Step into the Future of Manufacturing RNA
Evolve beyond traditional chemistry with top-tier enzymatic siRNA synthesis.
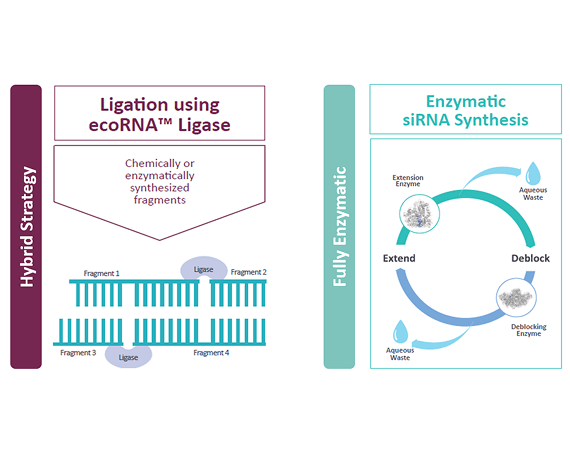
When manufacturing siRNA for therapeutic applications, Codexis delivers high yields and minimizes unwanted byproducts, while being economically sound. We have a suite of enzymatic pathways to synthesize siRNA, so that you can choose an option that best meets your needs.
The ECO Synthesis™ manufacturing platform (Enzyme-Catalyzed Oligonucleotide Synthesis) is an enzymatic, two-step process that streamlines process development while delivering higher purity siRNA through a scalable, efficient pathway. The RNA Ligase Screening and Optimization Services enables a hybrid approach, where engineered dsRNA (double-stranded RNA) ligases are used to join multiple short, single-stranded RNA (ssRNA) fragments together to form the desired dsRNA duplex.
Partner with Codexis for your siRNA manufacturing needs. We bring:


Our enzymatic tools and platforms enable the scalable and sustainable manufacture of small molecule API and siRNA for our customers while reducing costs and time of manufacturing. Contact us today to learn more about how we can scale your capabilities.
Contact Us