S-1/A: General form of registration statement for all companies including face-amount certificate companies
Published on February 1, 2010
Table of Contents
As filed with the Securities and Exchange Commission on February 1, 2010
Registration No. 333-164044
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 2
TO
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
CODEXIS, INC.
(Exact name of Registrant as specified in its charter)
| Delaware | 8731 | 71-0872999 | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
200 Penobscot Drive, Redwood City, CA 94063
(650) 421-8100
(Address, including zip code, and telephone number, including area code, of Registrants principal executive offices)
Douglas T. Sheehy
Senior Vice President, General Counsel and Secretary
Codexis, Inc.
200 Penobscot Drive
Redwood City, CA 94063
(650) 421-8100
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| Patrick A. Pohlen Gregory Chin Latham & Watkins LLP 140 Scott Drive Menlo Park, CA 94025 Telephone: (650) 328-4600 Facsimile: (650) 463-2600 |
John A. Fore Michael S. Russell Wilson Sonsini Goodrich & Rosati, Professional Corporation 650 Page Mill Road Palo Alto, CA 94304 Telephone: (650) 493-9300 Facsimile: (650) 493-6811 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of large accelerated filer, accelerated filer and smaller reporting company in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ¨ | Accelerated filer ¨ | |||
| Non-accelerated filer (Do not check if a smaller reporting company) x | Smaller reporting company ¨ | |||
CALCULATION OF REGISTRATION FEE
| Title of Each Class of Securities to be Registered |
Proposed Maximum Aggregate Offering Price(1) |
Amount of Registration Fee(2) |
||
| Common Stock, $0.0001 par value |
$100,000,000 | $7,130 |
||
| (1) | Estimated solely for the purpose of computing the amount of the registration fee pursuant to Rule 457(o) under the Securities Act of 1933. Includes the offering price of additional shares that the underwriters have the option to purchase. |
| (2) | The registrant previously paid a registration fee of $3,930 with a registration statement on Form S-1, File No. 333-150224, initially filed with the Commission on April 14, 2008. Pursuant to Rule 457(p) of the Securities Act of 1933, $3,930 of the previously paid registration fee is offset against the registration fee otherwise due for this registration statement. The remaining balance of the registration fee, or $3,200, was previously paid in connection with the initial filing of this Registration Statement on December 28, 2009. |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information contained in this prospectus is not complete and may be changed. We and the selling stockholders may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and we and the selling stockholders are not soliciting offers to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED FEBRUARY 1, 2010
Shares

Codexis, Inc.
Common Stock
Prior to this offering, there has been no public market for our common stock. We anticipate that the initial public offering price will be between $ and $ per share. We have applied to list our common stock on The Nasdaq Global Market under the symbol CDXS.
We are selling shares of our common stock through the underwriters. The selling stockholders identified in this prospectus are offering an additional shares through the underwriters. We will not receive any of the proceeds from the sale of the shares being sold by the selling stockholders.
To the extent the underwriters sell more than shares of common stock, the underwriters have the option to purchase up to an additional shares from us, at the initial public offering price, less the underwriting discounts and commissions.
Investing in our common stock involves risks. See Risk Factors beginning on page 11.
| Price to Public | Underwriting Discounts and Commissions |
Proceeds to Codexis |
Proceeds to Selling Stockholders |
|||||||||
| Per Share |
$ | $ | $ | $ | ||||||||
| Total |
$ | $ | $ | $ | ||||||||
Delivery of the shares of common stock will be made on or about , 2010.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| Credit Suisse | Goldman, Sachs & Co. | |||
| Piper Jaffray | RBC Capital Markets | Pacific Crest Securities | ||
The date of this prospectus is , 2010.
Table of Contents
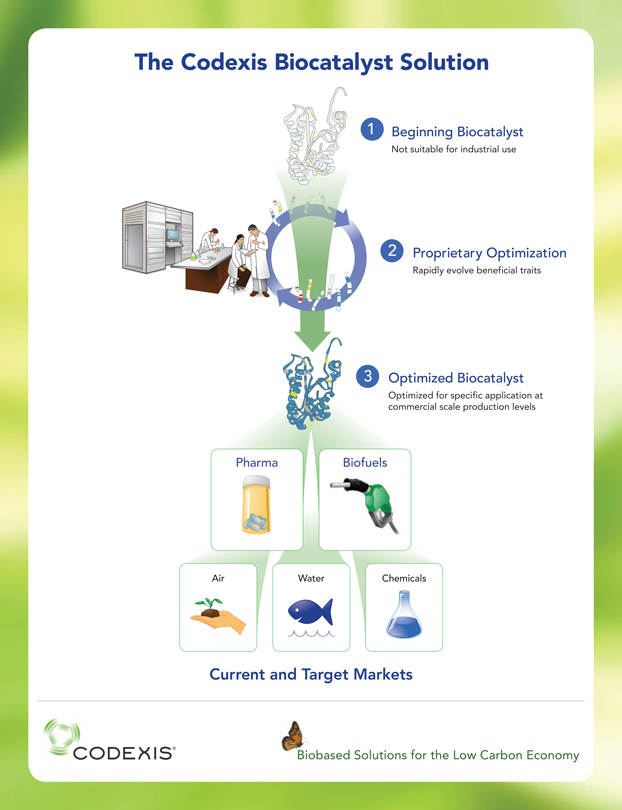
The Codexis Biocatalyst Solution
1 Beginning Biocatalyst
Not suitable for industrial use
2 Proprietary Optimization
Rapidly evolve beneficial traits
3 Optimized Biocatalyst
Optimized for specific application at commercial scale production levels
Pharma
Biofuels
Air
Water
Chemicals
Current and Target Markets
CODEXIS
Biobased Solutions for the Low Carbon Economy
Table of Contents
You should rely only on the information contained in this prospectus. We, the selling stockholders and the underwriters have not authorized anyone to provide you with information different from that contained in this prospectus. We and the selling stockholders are offering to sell, and seeking offers to buy, shares of common stock only in jurisdictions where offers and sales are permitted. The information contained in this prospectus is accurate only as of the date on the front cover of this prospectus, or such other dates as are stated in this prospectus, regardless of the time of delivery of this prospectus or of any sale of our common stock.
Dealer Prospectus Delivery Obligation
Until , 2010 (25 days after commencement of this offering), all dealers that buy, sell, or trade shares of our common stock, whether or not participating in this offering, may be required to deliver a prospectus. This delivery requirement is in addition to the obligation of dealers to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
i
Table of Contents
This summary highlights information contained elsewhere in this prospectus and does not contain all of the information you should consider in making your investment decision. You should read this summary together with the more detailed information, including our financial statements and the related notes, appearing elsewhere in this prospectus. You should carefully consider, among other things, the matters discussed in Risk Factors, before making an investment decision. Unless otherwise indicated herein, Codexis, Inc., Codexis, the Company, we, us and our refer to Codexis, Inc. and its subsidiaries.
Our Company
Our proprietary technology platform enables the creation of optimized biocatalysts that make existing industrial processes faster, cleaner and more efficient than current methods and has the potential to make new industrial processes possible at commercial scale. We have commercialized our biocatalysts in the pharmaceutical industry and are developing biocatalysts for use in producing advanced biofuels under a multi-year research and development collaboration with Shell. We are also using our technology platform to pursue biocatalyst-enabled solutions in other bioindustrial markets, including carbon management, water treatment and chemicals.
Biocatalysts are enzymes or microbes that initiate or accelerate chemical reactions. Manufacturers have historically used naturally occurring biocatalysts to produce many goods used in everyday life. However, inherent limitations in naturally occurring biocatalysts have restricted their commercial use. Our proprietary technology platform is able to overcome many of these limitations, allowing us to evolve and optimize biocatalysts to perform specific and desired chemical reactions at commercial scale.
We have focused our biocatalyst development efforts on large and rapidly growing markets, including pharmaceuticals and advanced biofuels. We have enabled biocatalyst-based drug manufacturing processes at commercial scale and have delivered biocatalysts and drug products to some of the worlds leading pharmaceutical companies, including Dr. Reddys Laboratories Ltd., Merck & Co., Inc., Pfizer Inc. and Ranbaxy Laboratories Limited. In our research and development collaboration with Shell, we are developing biocatalysts for use in producing advanced biofuels from renewable sources of non-food plant materials, known as cellulosic biomass.
The Biocatalysis Opportunity Industry Overview
Biocatalyst-enabled manufacturing processes may address a number of the drawbacks of conventional chemistry-based manufacturing. For example, unlike most chemistry-based manufacturing processes, biocatalysts can operate at or near room temperature and pressure, and often use manufacturing equipment that is less complex and expensive to build and operate. Biocatalyst-enabled processes can create products with the same or higher quality as chemistry-based manufacturing processes, while reducing the risks associated with extreme manufacturing environments and without generating the high volumes of waste, some of it hazardous to health and the environment, typically associated with conventional chemistry-based manufacturing processes.
In addition, due to concerns about the environment and the scarcity and security of supply of petroleum, there is an increasing interest in using cellulosic biomass as the feedstock for a variety of products, including advanced biofuels and other chemicals, as a replacement for petroleum. To date, conventional chemistry-based manufacturing approaches have not resulted in commercially viable processes for the conversion of cellulosic biomass to biofuels and other products. Biocatalysts have the potential to enable processes for the development of products, such as cellulose-derived biofuels, that cannot currently be manufactured using alternative techniques.
Despite their potentially significant advantages, biocatalysts have not achieved their full potential in industrial applications. Naturally occurring biocatalysts are often not stable enough to be used in industrial
1
Table of Contents
settings, where conditions may differ significantly from those in the biocatalysts natural environments. The activity and productivity of these biocatalysts is often too limited to be cost-effective in commercial scale manufacturing. In addition, the activity of natural biocatalysts is typically inhibited by the end product of the reactions they facilitate. This characteristic of natural biocatalysts, which is referred to as product inhibition, results in limited product yields in industrial settings. Moreover, for certain industrial applications, there are no known naturally occurring biocatalysts that catalyze the desired reaction.
Due to these limitations, other companies and researchers have tried to improve the performance of naturally occurring biocatalysts by directing their evolution through biotechnology techniques such as the random mutation of genes. However, to date, these techniques have had only limited success for a number of reasons. For example, random mutations of genes often result in decreased, not improved, performance and these alternative biotechnology techniques cannot effectively remove accumulated detrimental mutations. The end result is often an evolved biocatalyst with activity that reaches a plateau at a level that is insufficient for a commercial process. We believe there is a significant opportunity for novel technologies that can address the limitations of other biotechnology techniques and can substantially enhance the performance of biocatalysts in industrial settings.
Our Platform Technology
We believe that our proprietary technology platform can transform the industrial application of biocatalysts by improving their commercially relevant characteristics, such as stability, activity, product yield and tolerance to industrial conditions, while reducing product inhibition. In addition, our technology platform allows us to develop and optimize biocatalysts much more rapidly than is currently possible with alternative methods. Perhaps most importantly, we have demonstrated that our technology platform can enable the manufacture of products cost-effectively, at commercial scale and with significantly reduced environmental impact relative to conventional manufacturing processes.
Our proprietary technology platform uses advanced biotechnology methods, bioinformatics and years of accumulated know-how to significantly expedite the process of developing optimized biocatalysts. Key components of our technology platform include gene shuffling, whole genome shuffling, multiplexed gene SOEing, and proprietary bioinformatic software tools that allow us to identify and quantify the potential value of beneficial mutations and avoid detrimental mutations.
Our Target Markets and Solutions
Pharmaceuticals
Our technology platform enables us to deliver solutions to our customers in the pharmaceutical market by developing and delivering optimized biocatalysts that perform chemical transformations at a lower cost, and improve the efficiency and productivity of manufacturing processes. We provide value throughout the pharmaceutical product lifecycle, from preclinical development to clinical development and commercialization of products and the eventual transition from branded to generic products. Our technology platform allows us to provide benefits to our customers in a number of ways, including:
| | reducing the use of raw materials and intermediate products; |
| | improving product yield; |
| | using water as a primary solvent; |
| | performing reactions at or near room temperature and pressure; |
| | eliminating the need for certain costly manufacturing equipment; |
| | reducing energy requirements; |
| | reducing the need for late-stage purification steps; |
2
Table of Contents
| | eliminating multiple steps in the manufacturing process; and |
| | eliminating hazardous inputs and harmful emission by-products. |
Early in the product lifecycle, customers can use our services to achieve speed to market and to reduce manufacturing costs. If a pharmaceutical company that has developed a patent-protected drug, known as an innovator, incorporates our products or processes into an FDA-approved product, we expect the innovator to continue to use these products or processes for the patent life of the approved drug.
After a product is launched, customers also use our services to reduce manufacturing costs. At this stage, changes in the manufacturing process originally approved by the FDA may require additional review. Typically, pharmaceutical companies will only seek FDA approval for a manufacturing change if there are substantial cost savings associated with the change. We believe that the cost savings associated with our products may lead our customers to change their manufacturing processes for approved products and, if necessary, seek FDA approval of the new processes which incorporate our biocatalysts. Moreover, we believe these cost savings are attractive to generics manufacturers, who compete primarily on price.
Our products and services include our Codex Biocatalyst Panels, biocatalyst screening services, biocatalyst optimization services, biocatalysts and intermediates and active pharmaceutical ingredients, or APIs.
Biofuels
We believe that our technology platform will enable the development of biocatalysts that can be used to produce commercially viable, cellulose-derived biofuel alternatives to petroleum-based fuels. Since 2006, we have been engaged with Equilon Enterprises LLC dba Shell Oil Products US, which we refer to as Shell, in a research and development collaboration under which we are developing biocatalysts for use in producing advanced biofuels. Advanced biofuels are liquid transportation fuels derived from non-food biomass and which meet certain minimum carbon reduction criteria. Under the Energy Independence and Security Act of 2007, a minimum of 21 billion gallons of advanced biofuels must be sold in the United States by 2022. Our advanced biofuels program focuses on two primary elements: (1) developing biocatalysts to convert cellulosic biomass into sugars; and (2) converting these sugars into two advanced biofuels, cellulosic ethanol and biohydrocarbon diesel. For the first element, we have used our technology platform to improve our cellulase and other biocatalysts. For the second element, we have developed a biocatalyst that converts sugars to diesel fuel, and are working on improving ethanol-producing yeast. We are using our technology platform to develop biocatalysts that we believe will:
| | increase the rate at which cellulosic biomass is converted into biofuels; |
| | increase the yield of biofuels produced from cellulosic biomass; |
| | eliminate the need to use food resources for the production of biofuels; |
| | provide producers with more flexibility in designing processes to convert cellulosic biomass to biofuels, thereby reducing the costs associated with building and operating biofuel production facilities; and |
| | enable the production of new types of cellulosic biofuels that could be alternatives to petroleum-based fuels. |
Under our research and development collaboration with Shell, Shell will have the right, but not the obligation, to commercialize any technology that we develop in our biofuels program. If Shell commercializes our biofuels technology, we will collect a royalty for every gallon of fuel that Shell produces using our technology. If Shell chooses to commercialize any biofuels products developed through our collaboration, we believe that the combination of our technology platform with Shells proven project development capabilities and resources could enable a biofuels solution that extends from the conversion of cellulosic biomass into biofuels to delivery and distribution of refined biofuels to consumers at the pump.
3
Table of Contents
Additional Bioindustrial Opportunities
We believe that our technology platform, together with the knowledge and experience gained from our efforts in the pharmaceutical market and in our biofuels development program, will allow us to capitalize on opportunities in other bioindustrial markets, including carbon management, water treatment and chemicals. Depending on the market, we may pursue collaborations with industry leaders to allow us to leverage their competitive strengths and resources in pursuit of these opportunities.
Our Business Model
Our business model allows us to simultaneously pursue multiple commercial opportunities across a number of major markets. Our business model has resulted in a diversified revenue stream that is predictable over the near term with significant growth potential, while allowing us to share risk with and leverage the capabilities of our collaborators. Our business model includes the following key elements:
| | Targeting Multiple Major and Growing Markets. We currently use our technology platform to produce biocatalysts that are used at commercial scale in the pharmaceutical market. Through our collaboration with Shell, we are developing biocatalysts for use in producing commercially viable biofuels from cellulosic biomass. We also believe that we can use our technology platform to deliver biocatalyst-enabled solutions to other bioindustrial markets, including carbon management, water treatment and chemicals. |
| | Capital-Efficient Collaborations with Industry Leaders. We have adopted a business model that leverages our collaborators engineering, manufacturing and commercial expertise, their distribution infrastructure and their ability to fund commercial scale production facilities. For instance, in the pharmaceuticals market, our supply relationship with Arch enables us to bring intermediates and/or APIs for branded pharmaceutical products to market with very limited additional capital. In addition, if we are able to develop biocatalysts that enable the commercial production of biofuels derived from cellulosic biomass and Shell decides to commercialize products based on this technology, we would need to rely on Shell, or other parties selected by Shell, to design and build the commercial scale fuel production facilities and to distribute the final fuel product. |
| | Diversified Revenue Base. We are generating a revenue stream that is diversified across distinct industries, which should mitigate our exposure to cyclical downturns or fluctuations in any one market. In 2008, our revenues were derived from the pharmaceuticals and biofuels markets, and consisted primarily of collaborative research and development revenues and product sales. We are pursuing biocatalyst-enabled solutions in other bioindustrial markets, including carbon management, water treatment and chemicals that, if successful, will allow us to further diversify our revenues. |
| | Visible and Predictable Revenues. Based on our existing arrangements, we believe that the revenues from both our biofuels and pharmaceutical businesses should be predictable over the near term. We receive bi-monthly payments from Shell that are based on the number of funded full-time employee equivalents, or FTEs, that work on our research collaboration with Shell. The number of funded FTEs that work on the program, and the payments from Shell for these FTEs, are specified in our collaborative research agreement, subject to Shells ability to increase or reduce the number of FTEs under certain conditions over time. Because we allow our pharmaceutical customers to achieve significant cost savings in their manufacturing processes, historically they have continued using our biocatalysts once they have begun using our biocatalyst-enabled process. |
4
Table of Contents
Strategy
Our objective is to be the leading provider of optimized biocatalyst-enabled solutions across a wide range of industries. Key elements of our strategy are as follows:
| | Become a leading biocatalyst supplier to the advanced biofuels market. Our primary development efforts are focused on producing biocatalysts that can enable Shell to become a global leader in the advanced biofuels market. We continue to build upon our milestone-driven, multi-year research and development collaboration with Shell as we advance our efforts to produce biofuels from cellulosic biomass cost-effectively at commercial scale. Because of our success to date, Shell has expanded our collaboration twice, which we believe positions us to be a key contributor to their overall biofuels strategy. |
| | Expand into new bioindustrial markets. We are actively pursuing opportunities in other bioindustrial markets, including through self-funded research in carbon management and the pursuit of funded collaborations in carbon management, water treatment and chemicals. We have the right to use the intellectual property developed in our collaboration with Shell in fields outside of fuels and related products. We intend to leverage this and other intellectual property and our technology platform to develop products in our other target markets. |
| | Continue growing our pharmaceutical business. We intend to pursue new collaborations in the pharmaceutical industry to integrate our products and services more deeply into drug development and manufacturing processes for clinical stage and commercially approved pharmaceutical products. As part of that effort, we will continue to aggressively market our Codex Biocatalyst Panels to pharmaceutical companies to demonstrate the capabilities of our technology platform. |
| | Secure access to additional production capacity. To increase our biocatalyst manufacturing capacity and establish secondary supply sources, we are working to establish long-term supply contracts with contract manufacturers and are evaluating whether to invest in our own manufacturing capabilities. We may also opportunistically seek to secure specialty manufacturing assets and expand existing relationships for the supply of our biocatalysts and key pharmaceutical intermediates and APIs. For example, in August 2008, we entered into an expanded supply relationship with Arch for the manufacture of intermediates and APIs for specified pharmaceutical products. |
| | Expand our business and technology platform through the addition of new technologies, products or businesses. In the past, we have expanded our business by acquiring companies with synergistic business plans and licensing new technology. We will continue to evaluate opportunities to acquire or license new technologies, products or businesses that complement or expand our capabilities, including in the carbon management, water treatment and chemical markets. In addition, we intend to continue to advance our technology platform by investing in our research and development capabilities to allow us to more rapidly identify and develop products and pursue new market opportunities. |
Corporate Information
We were incorporated in Delaware in January 2002 as a wholly-owned subsidiary of Maxygen, Inc. We commenced independent operations in March 2002, after licensing core enabling technology from Maxygen. As of September 30, 2009, Maxygen beneficially owned approximately 21.6% of our common stock. Our other investors include industry leaders such as Shell, Chevron Corporation, Pfizer and The General Electric Company. Our principal executive offices are located at 200 Penobscot Drive, Redwood City, CA 94063, and our telephone number is (650) 421-8100. Our website address is www.codexis.com. Information contained on our website is not incorporated by reference into this prospectus, and you should not consider information contained on our website to be part of this prospectus.
5
Table of Contents
Our logo, Codexis, Codex and Codex Biocatalyst Panel and other trademarks or service marks of Codexis, Inc. appearing in this prospectus are the property of Codexis, Inc. This prospectus contains additional trade names, trademarks and service marks of other companies. We do not intend our use or display of other companies trade names, trademarks or service marks to imply relationships with, or endorsement or sponsorship of us by, these other companies.
6
Table of Contents
The Offering
| Common stock offered by Codexis |
shares (or shares if the underwriters exercise their option to purchase additional shares in full). |
| Common stock offered by the selling stockholders |
shares. |
| Common stock to be outstanding after this offering |
shares (or shares if the underwriters exercise their option to purchase additional shares in full). |
| Proposed Nasdaq Global Market symbol |
CDXS |
| Use of proceeds |
We intend to use the net proceeds from this offering for working capital and other general corporate purposes, including the costs associated with being a public company. We may also use a portion of the net proceeds to acquire other businesses, products or technologies, and to increase our internal biocatalyst production capacity. However, we do not have agreements or commitments for any specific acquisitions at this time. We will not receive any proceeds from the sale of shares by the selling stockholders. Please see Use of Proceeds. |
| Risk factors |
See Risk Factors starting on page 11 of this prospectus for a discussion of factors you should carefully consider before deciding to invest in our common stock. |
The number of shares of common stock to be outstanding after this offering is based on 41,599,768 shares outstanding as of September 30, 2009 and excludes:
| | 10,962,854 shares of common stock issuable upon the exercise of options outstanding as of September 30, 2009 at a weighted average exercise price of $3.25 per share; |
| | 491,513 shares of common stock issuable upon the exercise of warrants outstanding as of September 30, 2009 at a weighted average exercise price of $3.95 per share; and |
| | shares of common stock reserved for issuance under our 2010 Equity Incentive Award Plan, which will become effective in connection with the consummation of this offering (plus an additional 3,226,648 shares of common stock reserved for future grant or issuance under our 2002 Stock Plan as of September 30, 2009, which shares will be added to the shares to be reserved under our 2010 Equity Incentive Award Plan upon the effectiveness of the 2010 Equity Incentive Award Plan). |
Except as otherwise indicated, all information in this prospectus assumes:
| | the conversion of all of our outstanding shares of preferred stock into 37,624,216 shares of common stock in connection with the consummation of this offering and the related conversion of all outstanding preferred stock warrants into common stock warrants; |
| | no exercise of the underwriters option to purchase additional shares; |
| | an amendment to certain of our preferred stock financing documents prior to the consummation of this offering; and |
| | the filing of our amended and restated certificate of incorporation, which will occur in connection with the consummation of this offering. |
7
Table of Contents
We refer to our Series A, Series B, Series C, Series D, Series E and Series F preferred stock collectively as redeemable convertible preferred stock for financial reporting purposes and in the financial tables included in this prospectus, as more fully explained in Note 2 to our consolidated financial statements. In other parts of this prospectus, we refer to our Series A, Series B, Series C, Series D, Series E and Series F preferred stock collectively as preferred stock.
8
Table of Contents
Summary Consolidated Financial Data
The following table sets forth a summary of our historical consolidated financial data for the periods ended or as of the dates indicated. We have derived the consolidated statements of operations data for the years ended December 31, 2006, 2007 and 2008 from our audited consolidated financial statements appearing elsewhere in this prospectus. We have derived the consolidated statements of operations data for the nine months ended September 30, 2008 and 2009 and the consolidated balance sheet data as of September 30, 2009 from our unaudited financial statements appearing elsewhere in this prospectus. You should read this table together with our consolidated financial statements and the accompanying notes, Selected Consolidated Financial Data and Managements Discussion and Analysis of Financial Condition and Results of Operations appearing elsewhere in this prospectus. The unaudited interim financial statements have been prepared on the same basis as the annual consolidated financial statements and, in the opinion of management, reflect all adjustments, which include only normal recurring adjustments, necessary to state fairly our financial position as of September 30, 2009 and results of operations and cash flows for the nine months ended September 30, 2008 and 2009. The summary consolidated financial data in this section is not intended to replace our consolidated financial statements and the accompanying notes. Our historical results are not necessarily indicative of our future results.
The following table also sets forth summary unaudited pro forma and pro forma as adjusted consolidated financial data, which gives effect to the transactions described in the footnotes to the table. The unaudited pro forma and pro forma as adjusted consolidated financial data is presented for informational purposes only and does not purport to represent what our consolidated results of operations or financial position actually would have been had the transactions reflected occurred on the dates indicated or to project our financial condition as of any future date or results of operations for any future period.
| Years Ended December 31, | Nine Months Ended September 30, |
|||||||||||||||||||
| 2006 | 2007 | 2008 | 2008 | 2009 | ||||||||||||||||
| (in thousands, except per share amounts) | ||||||||||||||||||||
| Consolidated Statements of Operations Data: |
||||||||||||||||||||
| Revenues: |
||||||||||||||||||||
| Product |
$ | 2,544 | $ | 11,418 | $ | 16,860 | $ | 10,777 | $ | 13,401 | ||||||||||
| Related party collaborative research and development |
863 | 8,481 | 30,239 | 18,174 | 43,963 | |||||||||||||||
| Collaborative research and development |
8,403 | 4,733 | 3,062 | 2,678 | 1,295 | |||||||||||||||
| Government grants |
317 | 701 | 317 | 251 | 11 | |||||||||||||||
| Total revenues |
12,127 | 25,333 | 50,478 | 31,880 | 58,670 | |||||||||||||||
| Costs and operating expenses: |
||||||||||||||||||||
| Cost of product revenues |
1,806 | 8,319 | 13,188 | 7,922 | 11,886 | |||||||||||||||
| Research and development |
17,257 | 35,644 | 45,554 | 33,250 | 39,486 | |||||||||||||||
| Selling, general and administrative |
11,880 | 19,713 | 35,709 | 28,300 | 20,939 | |||||||||||||||
| Total costs and operating expenses |
30,943 | 63,676 | 94,451 | 69,472 | 72,311 | |||||||||||||||
| Loss from operations |
(18,816 | ) | (38,343 | ) | (43,973 | ) | (37,592 | ) | (13,641 | ) | ||||||||||
| Interest income |
742 | 1,491 | 1,538 | 1,435 | 141 | |||||||||||||||
| Interest expense and other, net |
(724 | ) | (2,533 | ) | (2,365 | ) | (2,517 | ) | (1,530 | ) | ||||||||||
| Loss before provision (benefit) for income taxes |
(18,798 | ) | (39,385 | ) | (44,800 | ) | (38,674 | ) | (15,030 | ) | ||||||||||
| Provision (benefit) for income taxes |
(127 | ) | (408 | ) | 327 | 126 | 79 | |||||||||||||
| Net loss |
$ | (18,671 | ) | $ | (38,977 | ) | $ | (45,127 | ) | $ | (38,800 | ) | $ | (15,109 | ) | |||||
| Net loss per share of common stock, basic and diluted |
$ | (10.99 | ) | $ | (15.53 | ) | $ | (12.64 | ) | $ | (11.02 | ) | $ | (3.86 | ) | |||||
| Weighted average common shares used in computing net loss per share of common stock, basic and diluted |
1,699 | 2,510 | 3,570 | 3,521 | 3,917 | |||||||||||||||
| Net loss used in computing pro forma net loss per share of common stock, basic and diluted (unaudited)(1) |
$ | (45,230 | ) | $ | (14,760 | ) | ||||||||||||||
| Pro forma net loss per share of common stock, basic and diluted (unaudited)(1) |
$ | (1.26 | ) | $ | (0.37 | ) | ||||||||||||||
| Weighted average common shares used in computing pro forma net loss per share of common stock, basic and diluted (unaudited)(1) |
35,900 | 39,684 | ||||||||||||||||||
9
Table of Contents
| (1) | Net loss used in computing pro forma basic and diluted net loss per share of common stock, pro forma basic and diluted net loss per share of common stock and number of weighted average common shares used in computing pro forma basic and diluted net loss per share of common stock in the table above give effect to the automatic conversion of all of our outstanding redeemable convertible preferred stock into common stock upon the closing of this offering as if such conversion had occurred at the beginning of each period or upon issuance, if later. |
| September 30, 2009 | |||||||||
| Actual | Pro Forma(1) | Pro Forma As Adjusted(2)(3) |
|||||||
| (in thousands) | |||||||||
| Consolidated Balance Sheet Data: |
|||||||||
| Cash, cash equivalents and marketable securities |
$ | 55,962 | $ | 55,962 | |||||
| Working capital |
28,061 | 29,792 | |||||||
| Total assets |
93,207 | 93,207 | |||||||
| Redeemable convertible preferred stock warrant liability |
1,731 | | |||||||
| Current and long-term financing obligations |
9,505 | 9,505 | |||||||
| Redeemable convertible preferred stock |
177,746 | | |||||||
| Stockholders (deficit) equity |
(140,893 | ) | 38,584 | ||||||
| (1) | The pro forma consolidated balance sheet data gives effect to (i) conversion of all of our outstanding shares of redeemable convertible preferred stock into shares of common stock, and (ii) conversion of all of our warrants for redeemable convertible preferred stock into warrants for common stock and the related reclassification of redeemable convertible preferred stock warrant liability to stockholders equity upon the completion of this offering. |
| (2) | The pro forma as adjusted consolidated balance sheet data gives effect to the sale of shares of common stock in this offering at an assumed initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus), after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
| (3) | Each $1.00 increase or decrease in the assumed initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus) would increase or decrease, as applicable, our pro forma as adjusted cash, cash equivalents and marketable securities, working capital, total assets and stockholders equity by approximately $ million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
10
Table of Contents
Investing in our common stock involves a high degree of risk. You should carefully consider the following risk factors, as well as the other information in this prospectus, before deciding whether to invest in shares of our common stock. The occurrence of any of the events described below could harm our business, financial condition, results of operations and growth prospects. In such an event, the trading price of our common stock may decline and you may lose all or part of your investment.
Risks Relating to Our Business and Strategy
We have a limited operating history, which may make it difficult to evaluate our current business and predict our future performance.
Our company has been in existence since early 2002. From 2002 until 2005, our operations focused on organizing and staffing our company and developing our technology platform. In 2005, we recognized our first revenues from product sales. Since 2005, we have continued to generate revenues, but because our revenue growth has occurred in recent periods, our limited operating history may make it difficult to evaluate our current business and predict our future performance. Any assessments of our current business and predictions you make about our future success or viability may not be as accurate as they could be if we had a longer operating history. We have encountered and will continue to encounter risks and difficulties frequently experienced by growing companies in rapidly changing industries. If we do not address these risks successfully, our business will be harmed.
Our quarterly operating results may fluctuate in the future. As a result, we may fail to meet or exceed the expectations of research analysts or investors, which could cause our stock price to decline.
Our financial condition and operating results have varied significantly in the past and may continue to fluctuate from quarter to quarter and year to year in the future due to a variety of factors, many of which are beyond our control. Factors relating to our business that may contribute to these fluctuations include the following factors, as well as other factors described elsewhere in this prospectus:
| | our ability to achieve or maintain profitability; |
| | actions that could cause us to lose any of our rights under our license from Maxygen; |
| | our relationships with and dependence on collaborators in our principal markets; |
| | our dependence on Shell for the development and commercialization of biofuels; |
| | the feasibility of producing and commercializing biofuels derived from cellulose; |
| | our dependence on a limited number of customers; |
| | our dependence on a limited number of contract manufacturers of our biocatalysts and suppliers for our pharmaceutical intermediates and APIs; |
| | our ability to manage our growth; |
| | our pharmaceutical customers abilities to incorporate our biocatalysts into their manufacturing processes; |
| | the outcomes of clinical trials conducted by our innovator customers; |
| | our ability to develop and successfully commercialize new products for the pharmaceuticals market; |
| | the effect of consolidation in the pharmaceutical industry on demand for our products; |
| | our ability to commercialize our technology in other bioindustrial markets; |
| | our ability to maintain license rights for commercial scale expression systems for cellulases; |
11
Table of Contents
| | fluctuations in the price of and demand for petroleum-based fuels; |
| | the availability of non-food renewable cellulosic biomass sources; |
| | reductions or changes to existing fuel regulations and policies; |
| | the existence of government subsidies or regulation with respect to carbon dioxide emissions; |
| | our potential need for additional licenses from Maxygen to pursue certain future business opportunities in the chemical market; |
| | our ability to obtain and maintain governmental grants; |
| | risks associated with the international aspects of our business; |
| | our ability to integrate any businesses we may acquire with our business; |
| | potential issues related to our ability to accurately report our financial results in a timely manner; |
| | our dependence on, and the need to attract and retain, key management and other personnel; |
| | our ability to obtain, protect and enforce our intellectual property rights; |
| | our ability to prevent the theft or misappropriation of our biocatalysts, the genes that code for our biocatalysts, know-how or technologies; |
| | potential advantages that our competitors and potential competitors may have in securing funding or developing products; |
| | our ability to obtain additional capital that may be necessary to expand our business; |
| | business interruptions such as earthquakes and other natural disasters; |
| | public concerns about the ethical, legal and social ramifications of genetically engineered products and processes; |
| | our ability to comply with laws and regulations; |
| | our ability to properly handle and dispose of hazardous materials used in our business; |
| | potential product liability claims; and |
| | our ability to use our net operating loss carryforwards to offset future taxable income. |
Due to the various factors mentioned above, and others, the results of any prior quarterly or annual periods should not be relied upon as indications of our future operating performance.
We have a history of net losses, and we may not achieve or maintain profitability.
We have incurred net losses since our inception, including losses of $18.7 million, $39.0 million and $45.1 million in 2006, 2007 and 2008, respectively, and $15.1 million for the nine months ended September 30, 2009. As of September 30, 2009, we had an accumulated deficit of $154.4 million. We expect to incur losses and negative cash flow from operating activities for the foreseeable future. To date, we have derived a substantial portion of our revenues from research and development agreements with our collaborators and expect to derive a substantial portion of our revenues from these sources for the foreseeable future. If we are unable to extend our existing agreements or enter into new agreements upon the expiration or termination of our existing agreements, our revenues could be adversely affected. In addition, some of our collaboration agreements provide for milestone payments and future royalty payments, the payment of which are uncertain as they are dependent on our and our collaborators abilities and willingness to successfully develop and commercialize products. We expect to spend significant amounts to fund the development of additional pharmaceutical and potential bioindustrial products, including biofuels. As a result, we expect that our expenses will exceed revenues for the foreseeable future
12
Table of Contents
and we do not expect to achieve profitability during this period, if ever. If we fail to achieve profitability, or if the time required to achieve profitability is longer than we anticipate, we may not be able to continue our business. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis.
In each of the last two audits of our consolidated financial statements, we and our independent registered public accounting firm have identified a material weakness and other deficiencies in our internal control over financial reporting. If we fail to remediate the deficiencies in our control environment or are unable to implement and maintain effective internal control over financial reporting in the future, the accuracy and timeliness of our financial reporting may be adversely affected.
We have not performed an evaluation of our internal control over financial reporting, such as required by Section 404 of the Sarbanes-Oxley Act, nor have we engaged our independent registered public accounting firm to perform an audit of our internal control over financial reporting as of any balance sheet date or for any period reported in our financial statements. Had we performed such an evaluation or had our independent registered public accounting firm performed an audit of our internal control over financial reporting, control deficiencies, including material weaknesses and significant deficiencies, in addition to those discussed below, may have been identified.
Solely in connection with the audit of our consolidated financial statements for 2005, 2006 and 2007, we and our independent registered public accounting firm identified a material weakness in our internal control over financial reporting. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of a companys annual or interim financial statements will not be prevented or detected on a timely basis. The material weakness comprised (i) our lack of policies and procedures, with the associated internal controls, to appropriately address complex, non-routine transactions and (ii) the lack of a sufficient number of qualified personnel to timely account for such transactions in accordance with U.S. generally accepted accounting principles. We and our independent registered public accounting firm identified the following issues during the audit, which collectively gave rise to the conclusion that we had an underlying material weakness: (i) inadequate policies to address the increasingly complex revenue arrangements that we enter into; (ii) failure to correctly and timely identify all data required to evaluate the accounting impact of stock option grants and to recognize all requirements under applicable accounting standards; (iii) accounting issues that arose in connection with two acquisitions; (iv) improper recording of foreign currency cumulative translation adjustments; and (v) failure to effectively track and value inventory. These deficiencies in the design and operation of our internal controls resulted in the recording of numerous audit adjustments, and significantly delayed our financial statement close process, for the three year period ended December 31, 2007.
Solely in connection with the audit of our consolidated financial statements for 2008, we and our independent registered public accounting firm identified a material weakness and two significant deficiencies in our internal control over financial reporting. The material weakness related to an inadequately designed process to analyze and reconcile certain accounts and the failure of supervisors or business unit managers to review the analysis prepared for certain accounts. We and our independent registered public accounting firm identified the following issues during the audit, which collectively gave rise to the conclusion that we had an underlying material weakness: (i) duplicative accrual relating to legal expenses in our accounting records; (ii) incorrect inputs relating to the Black-Scholes calculation of fair value of non-employee stock options and warrants; (iii) under accrual of expenses for tax consulting work; (iv) incorrect input of costs eligible for reimbursement under a license agreement; (v) errors in the valuation of inventory; and (vi) failure to accrue penalties associated with foreign filing requirements. We and our independent registered public accounting firm also identified two significant deficiencies in our internal control over financial reporting relating to (i) misapplication of U.S. generally accepted accounting principles and (ii) an ineffective contract compliance process. The material weakness and significant deficiencies resulted in the recording of numerous audit adjustments.
13
Table of Contents
We have not yet been able to remediate the deficiencies identified in these audits. However, we have taken numerous steps and plan to take additional significant steps intended to address the underlying causes of
the material weaknesses and significant deficiencies in the immediate future, primarily through the development and implementation of formal policies, improved processes and documented procedures, the retention of third-party experts and contractors, and the hiring of additional accounting and finance personnel with technical accounting, inventory accounting and financial reporting experience. We do not know the specific timeframe needed to remediate all of the control deficiencies underlying these material weaknesses and significant deficiencies. In addition, we may incur significant incremental costs associated with this remediation, primarily due to the procurement, implementation and validation of robust accounting and financial reporting systems, the hiring of additional finance and accounting personnel and the continued use of third-party experts and contractors.
If we fail to enhance our internal control over financial reporting to meet the demands that will be placed upon us as a public company, including the requirements of the Sarbanes-Oxley Act, we may be unable to accurately report our financial results, or report them within the timeframes required by law or exchange regulations. In addition, while we currently rely on a third-party contractor to assist us in the preparation of our financial statements, we intend for our internal accounting and finance groups to handle our financial reporting obligations upon becoming a reporting company. We may encounter difficulties as we reduce our reliance on this contractor, which could impact our ability to timely and accurately prepare our financial statements. We cannot assure you that we will be able to remediate all of the control deficiencies underlying our existing material weaknesses or significant deficiencies in a timely manner, if at all, or that in the future additional material weaknesses or significant deficiencies will not exist or otherwise be discovered, a risk that is significantly increased in light of the complexity of our business and multinational operations, and the emerging need for complex inter-subsidiary transactions. If our efforts to remediate these control deficiencies are not successful or if other deficiencies occur, our ability to accurately and timely report our financial position, results of operations or cash flows could be impaired, which could result in late filings of our annual and quarterly reports under the Securities Exchange Act of 1934, as amended, restatements of our consolidated financial statements, a decline in our stock price, suspension or delisting of our common stock by The Nasdaq Global Market, or other material adverse effects on our business, reputation, results of operations, financial condition or liquidity.
If we lose our intellectual property rights licensed from Maxygen, we may be unable to continue our business.
We have licensed core enabling intellectual property rights and technology from Maxygen, Inc., or Maxygen, under our March 2002 license agreement with Maxygen, which was subsequently amended in September 2002, October 2002 and August 2006. Under the terms of the license agreement, we are obligated, among other things, to pay Maxygen a significant percentage of certain types of consideration we receive in connection with our biofuels research and development collaboration with Shell. As a result of consideration received in connection with this collaboration, we were obligated to pay Maxygen $7.8 million, $1.0 million and $4.2 million for 2007 and 2008 and for the nine months ended September 30, 2009, respectively.
We rely heavily on the technology licensed to us by Maxygen and third parties under the Maxygen license. This technology includes advanced biotechnology methods, bioinformatics and years of accumulated know-how to develop the biocatalysts that are central to our business. Certain technologies sublicensed to us from Maxygen are owned by third parties, and our use of these technologies may be restricted by Maxygens agreements with those third parties. Maxygen has the right to terminate our rights under the license with respect to fuels, but not with respect to chemicals or pharmaceuticals, if we breach our royalty obligations to Maxygen and do not cure such breach within 60 days after we receive notice of the breach. In addition, as part of the license we received from Maxygen, Maxygen assigned or sublicensed to us several license agreements between Maxygen and third parties, including an agreement with one of
14
Table of Contents
our competitors, Novozymes A/S, or Novozymes. These third party agreements may restrict our use of the licensed technology. If we breach one of these third party agreements and fail to cure such breach within the time period specified in such third party agreement, Maxygen has the right to terminate our license with respect to the subject matter covered by the applicable third party agreement. Maxygen also has the right to terminate our license with respect to any family of related patent applications if we fail to pay our share of costs for obtaining and maintaining a patent licensed to us by Maxygen more than three times within any three-year period. In addition, Maxygen has the first right to control prosecution, maintenance and enforcement of certain licensed intellectual property rights. If Maxygen is acquired by a third party or transfers to a third party some or all of the intellectual property rights that we have licensed, the acquirer may choose not to enforce the intellectual property rights on which our business relies, or may seek to enforce those rights ineffectively and have them invalidated, and our ability to develop and expand our business may be adversely impacted. Any termination of our license agreement with Maxygen or any of the rights licensed to us by third parties through Maxygen, or any loss of our intellectual property rights as a result of ineffective enforcement of such rights, would have a material adverse impact on our financial condition, results of operations and growth prospects and could prevent us from continuing our business.
The license agreement with Maxygen, the related sublicenses to third party technologies and the third party agreements assigned to us under the Maxygen agreement, and the interplay between those agreements, are highly complex. For example, the agreements rely on highly technical definitions and delineate permitted and restricted activities. As a result of this complexity, the agreements may be subject to differing interpretations by the counterparties that could lead to disputes or litigation, including for alleged breaches or claims that our products or activities are not covered by the scope of the licenses. If Maxygen or a third party were to make such a contention and we were unable to reach agreement on the meaning or scope of the licenses, we could be subject to litigation. Any such litigation may divert management time from focusing on business operations and could cause us to spend significant amounts of money. If such litigation were to be decided adversely to us, we could: lose our rights to utilize the subject intellectual property in our business; be forced to stop selling or using our products or processes that use the subject intellectual property; be required to obtain a license to use the subject intellectual property, which license may not be available on commercially reasonable terms, or at all; be forced to redesign those products or processes that use the subject intellectual property, which may result in significant cost or delay to us, or which could be technically infeasible; or be required to pay monetary damages.
Under our license with Maxygen, there are limitations on our ability to enforce Maxygens patents to which we hold a license, which could have a material adverse effect on our business.
Under our agreement with Maxygen, Maxygen has the first right to enforce many of the patents that we have licensed, particularly those directly related to gene shuffling technology. If Maxygen declines to enforce these patent rights, we can enforce these rights after a delay of up to six months, or Maxygen can deny us the ability to enforce if Maxygen concludes that such enforcement may have a material adverse impact on Maxygen or one or more other licensees of Maxygens technology. Some portions of the technology licensed to us by Maxygen are owned by third parties that retain the right to enforce the patents. If Maxygen or these third parties fail to enforce their patent rights, our business could be materially adversely affected. Maxygen also has the right to control the defense of patent infringement claims made by third parties alleging infringement related to gene shuffling technology. If Maxygen does not provide a timely and adequate defense to these claims, we could be forced to stop using the licensed technology, redesign our products and/or obtain a license from the party claiming infringement, which may not be available on commercially reasonable terms or at all. If Maxygen were to become acquired or controlled by a competitor of ours or a third party who is not willing to work with us on the same terms or commit the same resources as Maxygen, our business could be harmed.
15
Table of Contents
We are dependent on our collaborators, and our failure to successfully manage these relationships could prevent us from developing and commercializing many of our products and achieving or sustaining profitability.
Our ability to maintain and manage collaborations in our markets is fundamental to the success of our business. We currently have license agreements, research and development agreements, supply agreements and/or distribution agreements with various collaborators. We may have limited or no control over the amount or timing of resources that any collaborator is able or willing to devote to our partnered products or collaborative efforts. Any of our collaborators may fail to perform their obligations as expected. These collaborators may breach or terminate their agreements with us or otherwise fail to conduct their collaborative activities successfully and in a timely manner. Further, our collaborators may not develop products arising out of our collaborative arrangements or devote sufficient resources to the development, manufacture, marketing, or sale of these products. Moreover, disagreements with a collaborator could develop and any conflict with a collaborator could reduce our ability to enter into future collaboration agreements and negatively impact our relationships with one or more existing collaborators. If any of these events occur, or if we fail to maintain our agreements with our collaborators, we may not be able to commercialize our existing and potential products, grow our business, or generate sufficient revenues to support our operations. Our collaboration opportunities could be harmed if:
| | we do not achieve our research and development objectives under our collaboration agreements in a timely manner or at all; |
| | we develop products and processes or enter into additional collaborations that conflict with the business objectives of our other collaborators; |
| | we disagree with our collaborators as to rights to intellectual property we develop, or their research programs or commercialization activities; |
| | we are unable to manage multiple simultaneous collaborations; |
| | our collaborators become competitors of ours or enter into agreements with our competitors; |
| | our collaborators become unable or less willing to expend their resources on research and development or commercialization efforts due to general market conditions, their financial condition or other circumstances beyond our control; or |
| | consolidation in our target markets limits the number of potential collaborators. |
Additionally, our business could be negatively impacted if any of our collaborators or suppliers undergoes a change of control or were to otherwise assign the rights or obligations under any of our agreements. For example, under our license agreement with Shell, Shell may assign the agreement without our consent in connection with a change of control. If Shell or any of our other collaborators were to assign these agreements to a competitor of ours or to a third party who is not willing to work with us on the same terms or commit the same resources as the current collaborator, our business and prospects could be harmed.
Our future success is heavily dependent on our collaborative research agreement with Shell.
Our current business plan for biofuels is heavily dependent on our collaborative research agreement with Shell, which will continue to be critical to researching and developing successful biocatalysts for producing biofuel products. Shells efforts in commercializing those products profitably will be critical to the success of our business plan for biofuels. If we are unable to successfully execute on the development of products for Shell, our ability to expand into other bioindustrial areas may be significantly impaired, which will materially and adversely affect our ability to grow our business.
We cannot control the financial resources Shell devotes to our programs under the collaborative research agreement. Currently, we receive bi-monthly payments from Shell that are based on the number of
16
Table of Contents
full-time employee equivalents, or FTEs, that work on our research collaboration with Shell. The number of FTEs that work on the program, and the payments from Shell for these FTEs, are specified in our collaborative research agreement. Until November 1, 2010, Shell has the right to reduce the number of funded FTEs under the collaborative research agreement by up to FTEs following 60 days advance written notice. After November 1, 2010, Shell has the right to further reduce the number of funded FTEs, with any one reduction not to exceed funded FTEs, following advance written notice. The required notice period ranges from 30 to 270 days, so the earliest an FTE reduction could take place would be December 2, 2010. Following any such reduction, Shell is subject to a standstill period of between 90 and 360 days during which period Shell cannot provide notice of any further FTE reductions. The notice and standstill periods are dependent on the number of funded FTEs reduced, with the length of notice and standstill periods increasing commensurate with the number of FTEs reduced. Any such reduction would have a material adverse impact on our revenues and business plan for biofuels. Moreover, disputes may arise between us and Shell, which could delay the programs on which we are working or could prevent the commercialization of products developed under our research and development collaboration. If that were to occur, we may have to use funds, personnel, equipment, facilities and other resources that we have not budgeted to undertake certain activities on our own. Disagreements with Shell could also result in expensive arbitration or litigation, which may not be resolved in our favor. Performance issues, program delay or termination or unbudgeted use of our resources may have a material adverse effect on our business and financial condition. Even if we successfully develop commercially viable technologies, our ability to derive revenues from those technologies will be dependent upon Shells willingness and ability to commercialize them. Shell has the right, but not the obligation, to commercialize these technologies. If Shell decides to commercialize our technology, we would need to rely on Shell, or other parties selected by Shell, to design, finance and construct commercial scale biofuel facilities, and operate commercial scale facilities at costs that are competitive with traditional petroleum-based fuels and other alternative fuel technologies that may be developed. Shell could merge with or be acquired by another company or experience financial or other setbacks unrelated to our research collaboration agreement that could adversely affect us.
We have agreed to work exclusively with Shell until November 2012 in the field of converting cellulosic biomass into fermentable sugars that are used in the production of fuels and related products as well as the conversion of these sugars into fuels and related products. However, Shell is not required to work exclusively with us, and could develop or pursue alternative technologies that it decides to use for commercialization purposes instead of the technology developed under our collaborative research agreement with Shell. For example, Shell is currently working with Virent Energy Systems to develop a thermo-chemical approach to developing biogasoline. Even if Shell decides to commercialize products based on our technologies, they have no obligation to purchase their biocatalyst supply from us. If Shell does not pursue the commercialization of any cellulosic sugars, biofuels or related products that may be developed under our collaborative research agreement, our exclusive arrangement would prevent us from licensing any technology developed under the collaboration for the patent life of such technology, which could place us at a significant competitive disadvantage in the biofuels market.
We cannot guarantee that our relationship with Shell will continue. After November 1, 2010, Shell can terminate its collaborative research agreement with us for any or no reason by providing us with nine months notice. Each party also has the right to terminate the license agreement and the collaborative research agreement in the case of an uncured breach by the other party, and to terminate the collaborative research agreement if that party believes the other party has assigned the collaborative research agreement to a direct competitor of the terminating party. If our collaboration with Shell were to fail, we would likely need to find another collaborator to provide the financial assistance and infrastructure necessary for us to develop and commercialize our products and execute our strategy with respect to biofuels. Failure to maintain this relationship would have a material adverse effect on our business, financial condition and prospects.
17
Table of Contents
The success of our cellulosic ethanol program may be dependent on the performance of other parties.
In connection with our research and development collaboration with Shell, we entered into a multi-party collaborative research and license agreement with Iogen Energy Corporation, or Iogen, and Shell in July 2009, which is focused on developing technology to convert cellulosic biomass to ethanol for commercial scale production. Either Shell or Iogen may fail to perform their obligations under this collaboration, may breach or terminate the collaboration agreement or otherwise fail to conduct their collaborative activities successfully and in a timely manner. Further, they may not devote sufficient resources to the development of technology to convert cellulosic biomass to ethanol or may fail to develop the technology altogether. Moreover, disagreements or conflicts amongst the parties could develop and could negatively impact our development efforts or our relationships with Shell and Iogen. If any of these events occur, or if we fail to maintain this collaboration with Shell and Iogen, we may be unable to develop technology for use in the production of cellulosic ethanol at commercial scale, which would have an adverse impact on our ability to grow our business. In addition, the collaborative research and license agreement with Iogen and Shell terminates in the event (i) our separate license agreements with Shell terminate or (ii) Iogens separate technology license agreement with Shell terminates. In addition, Shell can terminate the collaborative research and license agreement for any or no reason by providing us and Iogen with 30 days notice. Any unilateral action by Shell to terminate either its separate license agreements with us or Iogen will prevent any further research and development activities under the multi-party collaboration. As a result, our ability to pursue research and development activities relating to the conversion of cellulosic biomass and our biofuels programs may be adversely impacted.
Production and commercialization of biofuels derived from cellulose may not be feasible.
We are developing biocatalysts for use in producing two advanced biofuels, cellulosic ethanol and biohydrocarbon diesel, as part of our research and development collaboration with Shell. However, production and commercialization of cellulosic biofuels may not be feasible for a variety of reasons. For example, the development of technology for converting sugar derived from non-food renewable biomass sources into a commercially viable biofuel is still in its early stages, and we do not know whether this can be done commercially or at all. To date, there has been limited private and government funding for research and development in advanced biofuels relative to the scope of the challenges presented by this development effort. Furthermore, there have been only a few well-directed public policies emphasizing investment in the research and development of, and providing incentives for the commercialization of and transition to, biofuels.
As of the date of this prospectus, we believe that there are no commercial scale cellulosic biofuel production plants in operation. There can be no assurance that anyone will be able or willing to develop and operate biofuel production plants at commercial scale or that any biofuel facilities can be profitable.
Additionally, different biocatalysts may need to be developed for use in different geographic locations to convert the cellulosic biomass available in each locale into sugars that can be used in the production of these biofuels. This will make the development of biofuels derived from cellulose more challenging and expensive.
Moreover, substantial development of infrastructure will be required for the ethanol market to grow. Areas requiring expansion include, but are not limited to, additional rail capacity, additional storage facilities for ethanol, increases in truck fleets capable of transporting ethanol within localized markets, expansion of refining and blending facilities to handle ethanol, and growth in the fleet of end user vehicles capable of using ethanol blends. Substantial investments required for infrastructure changes and expansions may not be made on a timely basis or at all. Any delay or failure in making the changes to or expansion of infrastructure could harm demand or prices for ethanol and impose additional costs that would hinder its commercialization.
18
Table of Contents
Finally, if existing tax credits, subsidies and other incentives in the United States and foreign markets are phased out or reduced, the overall cost of commercialization of cellulosic biofuels will increase.
We are dependent on a limited number of customers.
Our current revenues are derived from a limited number of key customers. For the year ended December 31, 2008, our top five customers accounted for 79% of our total revenues, with Shell alone accounting for 60% of our total revenues. For the nine months ended September 30, 2009, our top five customers accounted for 90% of our total revenues, with Shell accounting for 75% of our total revenues. We expect a limited number of customers to continue to account for a significant portion of our revenues for the foreseeable future. This customer concentration increases the risk of quarterly fluctuations in our revenues and operating results. The loss or reduction of business from one or a combination of our significant customers could materially adversely affect our revenues, financial condition and results of operations.
Our dependence on contract manufacturers for biocatalyst production exposes our business to risks.
We have limited internal capacity to manufacture biocatalysts and are unable to do so for commercial scale production. As a result, we are dependent upon the performance and capacity of third party manufacturers for the commercial scale manufacturing of our biocatalysts.
We rely on two primary contract manufacturers, CPC Biotech srl, or CPC, and Lactosan GmbH & Co. KG, or Lactosan, to manufacture substantially all of the commercial biocatalysts used in our pharmaceutical business. Our pharmaceutical business, therefore, faces risks of difficulties with, and interruptions in, performance by these contract manufacturers, the occurrence of which could adversely impact the availability, launch and/or sales of our enzymes in the future. We have qualified other contract manufacturers to manufacture biocatalysts for our pharmaceutical business, but we do not have agreements or commitments with such contract manufacturers at this time. The failure of any manufacturers that we may use to supply manufactured product on a timely basis or at all, or to manufacture our biocatalysts in compliance with our specifications or applicable quality requirements or in volumes sufficient to meet demand would adversely affect our ability to sell pharmaceutical products, could harm our relationships with our collaborators or customers and could negatively affect our revenues and operating results. For example, in 2008, we were required to secure an alternative source of certain biocatalysts when viruses infected one of our contract manufacturers facilities. If this or any similar event disrupts the operations of any of our suppliers in the future, we may be forced to secure alternative sources of supply, which may be unavailable on commercially acceptable terms, cause delays in our ability to deliver products to our customers, increase our costs and decrease our profit margins.
We do not currently have a long-term supply contract with CPC, Lactosan or any other contract manufacturers, which are under no obligation to manufacture our biocatalysts and could elect to discontinue their manufacture at any time. If we require additional manufacturing capacity and are unable to obtain it in sufficient quantity, we may not be able to increase our pharmaceutical sales, or we may be required to make substantial capital investments to build that capacity or to contract with other manufacturers on terms that may be less favorable than the terms we currently have with CPC or Lactosan. If we choose to build our own additional manufacturing capacity, it could take a year or longer before our facility is able to produce commercial volumes of our biocatalysts. In addition, if we contract with other manufacturers, we may experience delays of several months in qualifying them, which could harm our relationships with our collaborators or customers and could negatively affect our revenues or operating results.
We are working to establish long-term supply contracts with contract manufacturers and are evaluating whether to invest in our own manufacturing capabilities. However, we cannot guarantee that we will be able to enter into long-term supply contracts on commercially reasonable terms, or at all, or to
19
Table of Contents
acquire, develop or contract for internal manufacturing capabilities. Any resources we expend on acquiring or building internal manufacturing capabilities could be at the expense of other potentially more profitable opportunities.
We are primarily dependent on contract manufacturers to manufacture our pharmaceutical products.
We currently rely on a small number of contract manufacturers to manufacture all of our pharmaceutical intermediates and APIs. In particular, in August 2008, we entered into a series of agreements that significantly broadened our relationship with Arch, which serves as our exclusive supplier for certain intermediates and APIs, including atorvastatin and all intermediates used to manufacture atorvastatin.
Our pharmaceutical business may face risks of difficulties with, and interruptions in, performance by Arch, or any other contract manufacturer that we rely on to manufacture our intermediates and APIs, the occurrence of which could adversely impact the availability, launch and/or sales of our products in the future. Because we rely on Arch to supply us exclusively with certain intermediates and APIs, the failure of Arch to supply our products on a timely basis or at all, or to manufacture our products in compliance with our specifications or applicable quality requirements, which may include current Good Manufacturing Practices, or cGMP, or to manufacture these products in volumes sufficient to meet demand would adversely affect our ability to commercialize these products and could lead to lost sales and lost customer confidence and would negatively affect our revenues and operating results. If for any reason Arch is unable to meet our volume requirements, or if either we or Arch terminates our relationship prematurely pursuant to the terms of our agreements, we will need to contract with other suppliers. We may experience delays in contracting with other suppliers, or we may not be able to contract with other suppliers on commercially reasonable terms or at all. We will not have enough capacity to meet our current demand projections if we are faced with any such delay or inability to contract with other suppliers, which could adversely affect our ability to commercialize these products and could harm our relationships with our customers.
We also rely, to a lesser extent, on other contract manufacturers to supply other pharmaceutical intermediates. The failure of these manufacturers to supply intermediates, or to manufacture products in compliance with our specifications or in sufficient volumes, would have negative effects on our revenues and operating results.
We rely on Arch to market our products in certain regions, and Arch may not be able to effectively market our products.
Pursuant to our arrangements with Arch, Arch manufactures certain of our intermediates and APIs that we then sell in the United States, India, Europe, Canada and Israel, with Arch as our marketing partner in those countries. In the rest of the world, Arch will market and sell certain of our products, but we have agreed with Arch that, if Archs sales of our products exceed a certain dollar threshold in any one year in these territories, we will re-evaluate the payment structure for these sales and which party will continue to sell products in these territories. We must therefore rely on Arch for their financial resources and their marketing expertise for the commercialization of our products in these regions. We cannot control Archs level of activity or expenditure relating to the marketing of our products relative to the rest of their products or marketing efforts. Arch may fail to effectively market our products in these regions. Conflicting priorities, competing demands or other factors that we cannot control, and of which we may not be aware, may cause Arch to deemphasize our products. If we are unable to effectively leverage Archs marketing capabilities or Arch does not successfully promote our products in the designated territories as our sole marketing partner, this could harm our business, our revenues and operating results, and our ability to bring our products to the marketplace.
20
Table of Contents
We may continue to encounter difficulties managing our growth, which could adversely affect our business.
Our business has grown rapidly and we expect this growth to continue. Overall, we have grown from approximately 40 employees at the end of 2002 to approximately 300 employees as of September 30, 2009. Currently, we are working simultaneously on multiple projects targeting several markets. Furthermore, we are conducting our business across several countries, including activities in the United States, India, Japan, Singapore, Austria, France, Germany, Hungary and Italy. These diversified, global operations place increased demands on our limited resources and require us to substantially expand the capabilities of our administrative and operational resources and to attract, train, manage and retain qualified management, technicians, scientists and other personnel. As our operations expand domestically and internationally, we will need to continue to manage multiple locations and additional relationships with various customers, collaborators, suppliers and other third parties. Our ability to manage our operations, growth, and various projects effectively will require us to make additional investments in our infrastructure to continue to improve our operational, financial and management controls and our reporting systems and procedures and to attract and retain sufficient numbers of talented employees, which we may be unable to do effectively. As a result, we may be unable to manage our expenses in the future, which may negatively impact our gross margins or operating margins in any particular quarter. In addition, we may not be able to successfully improve our management information and control systems, including our internal control over financial reporting, to a level necessary to manage our growth and to remediate the existing material weaknesses and significant deficiencies in our internal control over financial reporting that were identified in our last two audits, and we may discover additional deficiencies in existing systems and controls that we may not be able to remediate in an efficient or timely manner.
Our business could be adversely affected if pharmaceutical customers do not incorporate our biocatalysts into their manufacturing processes.
Historically, pharmaceutical companies have been reluctant to use biocatalysts in the manufacture of their intermediates or APIs because naturally occurring biocatalysts were not economically viable for production at commercial scale. For example, naturally occurring biocatalysts are often not stable enough to be used in industrial settings. Additionally, the activity and productivity of these biocatalysts are often too limited to be effective in commercial scale manufacturing and often result in incomplete reactions and insufficient product yields. Although our biocatalysts have been developed to address shortcomings of naturally occurring biocatalysts, we may still encounter reluctance by pharmaceutical companies to adopt processes that use our biocatalysts. If customers decide not to adopt processes using our biocatalysts over other methods of producing the intermediates or APIs for their drugs, our revenues and prospects will be negatively impacted.
Moreover, we believe that the lower manufacturing costs enabled by our technology platform is one of the principal reasons pharmaceutical companies have purchased and will continue to purchase our biocatalysts and optimization services. If we are unable to maintain the cost advantages provided by our technology platform, customers may be less willing to purchase our products and services, which would also negatively impact our revenues. In addition, we may be unable to reach agreement on pricing or other terms with potential customers, which may adversely impact our ability to grow our business.
Our business could be adversely affected if the clinical trials being conducted by our innovator customers fail or if the processes used by those customers to manufacture their final pharmaceutical products fail to be approved.
Our biocatalysts are used in the manufacture of intermediates and APIs which are then used in the manufacture of final pharmaceutical products by our existing and potential customers who sell branded drugs, which we refer to as innovators. These pharmaceutical products must be approved by the FDA in the
21
Table of Contents
United States and similar regulatory bodies in other markets prior to commercialization. If these customers experience adverse events in their clinical trials, fail to receive regulatory approval for the drugs, or decide for business or other reasons to discontinue their clinical trials or drug development activities, our revenues and prospects will be negatively impacted. For example, one of our customers that incorporated our biocatalysts in the manufacturing process for a drug candidate suspended its development efforts during clinical trials. As a result, we were unable to realize a potential long-term revenue stream that would otherwise be associated with a commercialized product. The process of producing these drugs, and their generic equivalents, is also subject to regulation by the FDA in the United States and equivalent regulatory bodies in other markets. If any pharmaceutical process that uses our biocatalysts does not receive approval by the appropriate regulatory body or if customers decide not to pursue approval, our business could be adversely affected.
If we are unable to develop and commercialize new products for the pharmaceutical market, our business and prospects will be harmed.
We have launched several new intermediates and APIs for generic drugs, including Singulair and Cymbalta, in markets in which they are not patent protected, and plan to launch these same products in various other markets once the patent protection for each product in those other markets expires. In addition, we plan to launch other new intermediates and APIs in the future. These efforts are subject to numerous risks, including the following:
| | we may be unable to successfully develop the biocatalysts or manufacturing processes for our intermediates and APIs in a timely and cost-effective manner, if at all; |
| | we may face difficulties in transferring the developed technologies to Arch, or other contract manufacturers that we may use, for commercial scale production; |
| | Arch, or other contract manufacturers that we may use, may be unable to scale their manufacturing operations to meet the demand for these products and we may be unable to secure additional manufacturing capacity; |
| | generics manufacturers may not be willing to purchase these products from us on favorable terms, if at all; |
| | we may face product liability litigation, unexpected safety or efficacy concerns and product recalls or withdrawals; |
| | changes in laws or regulations relating to the pharmaceutical industry could cause us to incur increased costs of compliance or otherwise harm our business; |
| | negative publicity may affect doctor or patient confidence in the products; |
| | we may face pressure from existing or new competitive products; and |
| | we may face pricing pressures from existing or new competitors, some of which may benefit from government subsidies or other incentives in their local markets. |
In addition, our innovator customers may view us as competitors and be less willing to do business with us. Moreover, we may be subject to claims alleging that our pharmaceutical products violate the patent or other intellectual property rights of third parties, particularly in connection with any generic products on which the patent covering the branded drug is expiring. These claims could give rise to litigation, which may be costly and time-consuming and could divert managements attention. If we are unsuccessful in our defense of any such claims, we may lose our right to develop or manufacture the products, be required to pay monetary damages, or be required to enter into license agreements and pay
22
Table of Contents
substantial royalties. If one or more of these risks were to materialize, our future business, results of operations and financial condition could be materially adversely affected, and we may be unable to grow our business.
Consolidation in the pharmaceutical industry could adversely impact our business.
There has been significant consolidation in the pharmaceutical industry, including the recent mergers of Pfizer Inc. and Wyeth, Merck and Schering-Plough Corporation and F. Hoffman-La Roche Ltd. and Genentech Inc., and the acquisition of several generics businesses by Novartis AG, and this consolidation may continue in the future. When pharmaceutical companies merge, they often rationalize their product portfolios by eliminating competing product programs, resulting in fewer drug programs for certain target indications. As a result of this consolidation, there are fewer potential pharmaceutical customers and fewer drug development programs that could utilize our products and services to enhance drug manufacturing processes. For example, the consolidation of two pharmaceutical companies may lead the acquiring company to suspend or terminate development programs for certain product candidates for which we may have been providing or had the opportunity to provide biocatalysts, intermediates or APIs. This would lead to diminished demand for our products and services, which could adversely impact our business.
If we are unable to successfully commercialize our technology in other bioindustrial markets, we may be unable to grow our business.
In addition to biofuels, we expect to invest a significant amount of our future research and development efforts in other bioindustrial markets, including carbon management, water treatment and chemicals. Because we do not currently and may never possess the resources necessary to independently develop and commercialize all of the potential products that may result from our technologies, our ability to succeed in these target markets will likely depend on our ability to enter into collaboration agreements to develop and commercialize potential products. We intend to pursue such additional collaborations, but may be unable to do so on terms satisfactory to us, or at all. Even if we are able to enter into collaborations in one or more of these areas, the collaborations may be unsuccessful. Moreover, because we have limited financial and managerial resources, we will be required to prioritize our application of resources to particular development and commercialization efforts. Any resources we expend on one or more of these efforts could be at the expense of other potentially profitable opportunities. If we focus our efforts and resources on one or more of these areas and they do not lead to commercially viable products, our revenues, financial condition and results of operations could be adversely affected.
If we are unable to maintain license rights to a commercial scale expression system for enzymes that convert cellulosic biomass to sugars, our business may be materially adversely affected.
We entered into a license agreement with Dyadic International, Inc. and its affiliate, or Dyadic, in November 2008 to obtain access to an expression system that is capable of producing the necessary biocatalysts for the commercialization of cellulosic biofuels. Under the license agreement with Dyadic, we obtained a non-exclusive license under intellectual property rights of Dyadic relating to Dyadics proprietary fungal expression technology for the production of enzymes. We also obtained access to specified materials of Dyadic relating to such Dyadic technology. Our license is sublicenseable to Shell in the field of biofuels. Dyadic has the right to terminate our licenses under the license agreement if we challenge the validity of any of the patents licensed under the license agreement and for various other reasons. Our licenses, and access to such materials of Dyadic, under the license agreement will terminate as a result of any termination of the license agreement other than due to Dyadics material breach. If we are unable to maintain these rights on commercially reasonable terms or if the license agreement is terminated for any reason, we will need to buy or license this type of expression system from another party or develop this type of expression system ourselves, which may be difficult, costly and time consuming, in part
23
Table of Contents
because of the broad, existing intellectual property rights owned by Danisco A/S, Novozymes and others. If any of these events occur, our business may be materially adversely affected.
Fluctuations in the price of and demand for petroleum-based fuels may reduce demand for biofuels.
Biofuels are anticipated to be marketed as an alternative to petroleum-based fuels. Therefore, if the price of oil falls, any revenues that we generate from biofuel products could decline, and we may be unable to produce products that are a commercially viable alternative to petroleum-based fuels. Additionally, demand for liquid transportation fuels, including biofuels, may decrease due to economic conditions or otherwise.
The royalties that we may earn under our agreements with Shell are indexed to the price of oil and generally increase as the price of oil increases. However, the index is set based on average prices between November 2007 and the date of first commercial sale. Therefore, if prices fall, our revenues would be negatively impacted.
Our approach to the biofuels markets may be limited by the availability or cost of non-food renewable cellulosic biomass sources.
Our approach to the biofuels markets will be dependent on the availability and price of the cellulosic biomass that will be used to produce biofuels derived from cellulose. If the availability of cellulosic biomass decreases or its price increases, this may reduce the royalties that we collect from Shell and have a material adverse effect on our financial condition and operating results. At certain levels, prices may make these products uneconomical to use and produce.
The price and availability of cellulosic biomass may be influenced by general economic, market and regulatory factors. These factors include weather conditions, farming decisions, government policies and subsidies with respect to agriculture and international trade, and global demand and supply. The significance and relative impact of these factors on the price of cellulosic biomass is difficult to predict, especially without knowing what types of cellulosic biomass materials we may need to use.
Reductions or changes to existing fuel regulations and policies may present technical, regulatory and economic barriers, all of which may significantly reduce demand for biofuels.
The market for biofuels is heavily influenced by foreign, federal, state and local government regulations and policies concerning the petroleum industry. For example, in 2007, the U.S. Congress passed an alternative fuels mandate that currently calls for 13 billion gallons of liquid transportation fuels sold in 2010 to come from alternative sources, including biofuels, a mandate that grows to 36 billion gallons by 2022. Of this amount, a minimum of 21 billion gallons must be advanced biofuels. In the United States and in a number of other countries, these regulations and policies have been modified in the past and may be modified again in the future. Any reduction in mandated requirements for fuel alternatives and additives to gasoline may cause demand for biofuels to decline and deter investment in the research and development of biofuels. Market uncertainty regarding future policies may also affect our ability to develop new biofuels products or to license our technologies to third parties. Any inability to address these requirements and any regulatory or policy changes could have a material adverse effect on our biofuels business, financial condition and operating results. Our other potential bioindustrial products may be subject to additional regulations.
If governmental incentives or other actions targeted at limiting carbon emissions are not adopted, a broad market for carbon management solutions may not develop.
Our strategy with respect to carbon management, although still in the research phase, would likely require an expansion of the market for the management of carbon dioxide emissions prior to us being able to recognize significant revenues from our research and continuing expenditures of resources. The
24
Table of Contents
development of a significant market will likely depend on the adoption of government subsidies or other government regulation requiring companies to limit their carbon emissions. In the absence of such additional government subsidies or regulation, this market may not expand and we would not be able to generate significant revenues from our carbon management operations.
We may need additional licenses from Maxygen to pursue certain future business opportunities in the chemicals market.
Under our license agreement with Maxygen, we obtained exclusive rights to manufacture certain types of chemicals for specified purposes within particular fields. Should we desire to work on any chemicals that are outside the scope of these license rights, we may need to seek additional rights from Maxygen. Maxygen has no obligation to grant such rights to us and may choose not to license such rights to us on favorable terms, if at all. If we are unable to obtain rights to those additional areas, we may not be able to develop products or services or pursue collaborations in those areas, which could limit our ability to expand into the chemicals market.
Our government grants are subject to uncertainty, which could harm our business and results of operations.
We have received various government grants to complement and enhance our own resources. We may seek to obtain government grants and subsidies in the future to offset all or a portion of the costs of building additional manufacturing facilities and research and development activities. We cannot be certain that we will be able to secure any such government grants or subsidies. Any of our existing grants or new grants that we may obtain may be terminated, modified or recovered by the granting governmental body under certain conditions.
We may also be subject to audits by government agencies as part of routine audits of our activities funded by our government grants. As part of an audit, these agencies may review our performance, cost structures and compliance with applicable laws, regulations and standards. Funds available under grants must be applied by us toward the research and development programs specified by the granting agencies, rather than for all of our programs generally. If any of our costs are found to be allocated improperly, the costs may not be reimbursed and any costs already reimbursed may have to be refunded. Accordingly, an audit could result in an adjustment to our revenues and results of operations.
We face risks associated with our international business.
Significant portions of our operations are conducted outside of the United States and we expect to continue to have significant foreign operations in the foreseeable future. International business operations are subject to a variety of risks, including:
| | changes in or interpretations of foreign regulations that may adversely affect our ability to sell our products or repatriate profits to the United States; |
| | the imposition of tariffs; |
| | the imposition of limitations on, or increase of, withholding and other taxes on remittances and other payments by foreign subsidiaries or joint ventures; |
| | the imposition of limitations on genetically-engineered products or processes and the production or sale of those products or processes in foreign countries; |
| | currency exchange rate fluctuations; |
| | uncertainties relating to foreign laws and legal proceedings including tax and exchange control laws; |
25
Table of Contents
| | the availability of government subsidies or other incentives that benefit competitors in their local markets that are not available to us; |
| | economic or political instability in foreign countries; |
| | difficulties in staffing and managing foreign operations; and |
| | the need to comply with a variety of U.S. laws applicable to the conduct of overseas operations, including export control laws and the Foreign Corrupt Practices Act. |
We manufacture many of our pharmaceutical intermediates in India, which has stringent local regulations that make it difficult for money earned in India to be taken out of the country without being subject to Indian taxes. While our Indian subsidiary can make use of some of the funds we earn in India, these regulations may limit the amount of profits we can repatriate from operations in India.
If we engage in any acquisitions, we will incur a variety of costs and may potentially face numerous risks that could adversely affect our business and operations.
We have made acquisitions in the past, and if appropriate opportunities become available, we expect to acquire additional businesses, assets, technologies, or products to enhance our business in the future. In connection with any future acquisitions, we could:
| | issue additional equity securities which would dilute our current stockholders; |
| | incur substantial debt to fund the acquisitions; or |
| | assume significant liabilities. |
Acquisitions involve numerous risks, including problems integrating the purchased operations, technologies or products, unanticipated costs and other liabilities, diversion of managements attention from our core businesses, adverse effects on existing business relationships with current and/or prospective collaborators, customers and/or suppliers, risks associated with entering markets in which we have no or limited prior experience and potential loss of key employees. We do not have extensive experience in managing the integration process and we may not be able to successfully integrate any businesses, assets, products, technologies, or personnel that we might acquire in the future without a significant expenditure of operating, financial and management resources, if at all. The integration process could divert management time from focusing on operating our business, result in a decline in employee morale and cause retention issues to arise from changes in compensation, reporting relationships, future prospects or the direction of the business. Acquisitions may also require us to record goodwill and non-amortizable intangible assets that will be subject to impairment testing on a regular basis and potential periodic impairment charges, incur amortization expenses related to certain intangible assets, and incur large and immediate write offs and restructuring and other related expenses, all of which could harm our operating results and financial condition. In addition, we may acquire companies that have insufficient internal financial controls, which could impair our ability to integrate the acquired company and adversely impact our financial reporting. If we fail in our integration efforts with respect to any of our acquisitions and are unable to efficiently operate as a combined organization, our business and financial condition may be adversely affected.
We must rely on our suppliers, contract manufacturers and customers to deliver timely and accurate information in order to accurately report our financial results in the time frame and manner required by law.
We need to receive timely, accurate and complete information from a number of third parties in order to accurately report our financial results on a timely basis. We rely on third parties that sell our pharmaceutical products that are manufactured using our biocatalysts to provide us with complete and accurate information regarding revenues, costs of revenues and payments owed to us on a timely basis. In
26
Table of Contents
addition, we rely on suppliers and certain contract manufacturers, including Arch, to provide us with timely and accurate information regarding our inventories and manufacturing cost information, and we rely on current and former collaborators to provide us with product sales and cost saving information in connection with royalties owed to us. Any failure to receive timely information from one or more of these third parties could require that we estimate a greater portion of our revenues and other operating performance metrics for the period, which could cause our reported financial results to be incorrect. Moreover, if the information that we receive is not accurate, our financial statements may be materially incorrect and may require restatement, and we may not receive the full amount of revenues that we are entitled to under these arrangements. Although we typically have audit rights with these parties, performing such an audit could be harmful to our collaborative relationships, expensive and time consuming and may not be sufficient to reveal any discrepancies in a timeframe consistent with our reporting requirements.
If we lose key personnel, including key management personnel, or are unable to attract and retain additional personnel, it could delay our product development programs, harm our research and development efforts, and we may be unable to pursue collaborations or develop our own products.
Our business involves complex, global operations across a variety of markets and requires a management team and employee workforce that is knowledgeable in the many areas in which we operate. The loss of any key members of our management, including our Chief Executive Officer, Alan Shaw, or the failure to attract or retain other key employees who possess the requisite expertise for the conduct of our business, could prevent us from developing and commercializing our products for our target markets and entering into collaborations or licensing arrangements to execute on our business strategy. In addition, the loss of any key scientific staff, or the failure to attract or retain other key scientific employees, could prevent us from developing and commercializing our products for our target markets and entering into collaborations or licensing arrangements to execute on our business strategy. We may not be able to attract or retain qualified employees in the future due to the intense competition for qualified personnel among biotechnology and other technology-based businesses, particularly in the biofuels area, or due to the availability of personnel with the qualifications or experience necessary for our biofuels business. If we are not able to attract and retain the necessary personnel to accomplish our business objectives, we may experience staffing constraints that will adversely affect our ability to meet the demands of our collaborators and customers in a timely fashion or to support our internal research and development programs. In particular, our product and process development programs are dependent on our ability to attract and retain highly skilled scientists. Competition for experienced scientists and other technical personnel from numerous companies and academic and other research institutions may limit our ability to do so on acceptable terms. All of our employees are at-will employees, which means that either the employee or we may terminate their employment at any time.
Our planned activities will require additional expertise in specific industries and areas applicable to the products and processes developed through our technology platform or acquired through strategic or other transactions, especially in the end markets that we seek to penetrate. These activities will require the addition of new personnel, and the development of additional expertise by existing personnel. The inability to attract personnel with appropriate skills or to develop the necessary expertise could impair our ability to grow our business. Additionally, we would be in breach of our collaborative research agreement with Shell if we fail to maintain a specified number of personnel.
Our ability to compete may decline if we do not adequately protect our proprietary technologies or if we lose some of our intellectual property rights through costly litigation or administrative proceedings.
Our success depends in part on our ability to obtain patents and maintain adequate protection of our intellectual property for our technologies and products and potential products in the United States and other countries. We have adopted a strategy of seeking patent protection in the United States and in foreign countries with respect to certain of the technologies used in or relating to our products and processes. As
27
Table of Contents
such, as of September 30, 2009, we owned or had licensed rights to approximately 235 issued patents and approximately 280 pending patent applications in the United States and in various foreign jurisdictions. Of the licensed patents and patent applications, most are owned by Maxygen and exclusively licensed to us for use with respect to certain products for specified purposes within certain fields. However, some of these patents will expire as early as 2014. As of September 30, 2009, we owned approximately 35 issued patents and approximately 110 pending patent applications in the United States and in various foreign jurisdictions. These patents and patent applications are directed to our enabling technologies and to our methods and products which support our business in the pharmaceuticals and bioindustrials markets. We intend to continue to apply for patents relating to our technologies, methods and products as we deem appropriate.
Numerous patents in our portfolio involve complex legal and factual questions and, therefore, enforceability cannot be predicted with any certainty. Issued patents and patents issuing from pending applications may be challenged, invalidated, or circumvented. Moreover, third parties could practice our inventions in territories where we do not have patent protection. Such third parties may then try to import products made using our inventions into the United States or other territories. Additional uncertainty may result from potential passage of patent reform legislation by the United States Congress, legal precedent as handed down by the United States Federal Circuit and Supreme Court as they determine legal issues concerning the scope and construction of patent claims and inconsistent interpretation of patent laws by the lower courts. Accordingly, we cannot ensure that any of our pending patent applications will result in issued patents, or even if issued, predict the breadth of the claims upheld in our and other companies patents. Given that the degree of future protection for our proprietary rights is uncertain, we cannot ensure that: (i) we were the first to make the inventions covered by each of our pending applications, (ii) we were the first to file patent applications for these inventions, and (iii) the proprietary technologies we develop will be patentable.
In addition, unauthorized parties may attempt to copy or otherwise obtain and use our products or technology. Monitoring unauthorized use of our intellectual property is difficult, and we cannot be certain that the steps we have taken will prevent unauthorized use of our technology, particularly in certain foreign countries where the local laws may not protect our proprietary rights as fully as in the United States. If competitors are able to use our technology, our ability to compete effectively could be harmed. Moreover, others may independently develop and obtain patents for technologies that are similar to or superior to our technologies. If that happens, we may need to license these technologies, and we may not be able to obtain licenses on reasonable terms, if at all, which could cause harm to our business.
Our commercial success also depends in part on not infringing patents and proprietary rights of third parties, and not breaching any licenses or other agreements that we have entered into with regard to our technologies, products and business. We cannot ensure that patents have not been issued to third parties that could block our ability to obtain patents or to operate as we would like. There may be patents in some countries that, if valid, may block our ability to commercialize products in those countries if we are unsuccessful in circumventing or acquiring the rights to these patents. There also may be claims in patent applications filed in some countries that, if granted and valid, may also block our ability to commercialize products or processes in these countries if we are unable to circumvent or license them.
28
Table of Contents
The biotechnology industry is characterized by frequent and extensive litigation regarding patents and other intellectual property rights, and we believe that the various bioindustrial markets will also be characterized by this type of litigation. Many biotechnology companies have employed intellectual property litigation as a way to gain a competitive advantage. Our involvement in litigation, interferences, opposition proceedings or other intellectual property proceedings inside and outside of the United States, to defend our intellectual property rights or as a result of alleged infringement of the rights of others, may divert management time from focusing on business operations and could cause us to spend significant amounts of money. Any potential intellectual property litigation also could force us to do one or more of the following:
| | stop selling, incorporating or using our products that use the subject intellectual property; |
| | obtain from the third party asserting its intellectual property rights a license to sell or use the relevant technology, which license may not be available on reasonable terms, or at all; or |
| | redesign those products or processes that use any allegedly infringing technology, which may result in significant cost or delay to us, or which could be technically infeasible. |
We are aware of a significant number of patents and patent applications relating to aspects of our technologies filed by, and issued to, third parties. We cannot assure you that if this third party intellectual property is asserted against us that we would ultimately prevail.
If any of our competitors have filed patent applications or obtained patents that claim inventions also claimed by us, we may have to participate in interference proceedings declared by the relevant patent regulatory agency to determine priority of invention and, thus, the right to the patents for these inventions in the United States. These proceedings could result in substantial cost to us even if the outcome is favorable. Even if successful, an interference may result in loss of certain claims. Any litigation or proceedings could divert our managements time and efforts. Even unsuccessful claims could result in significant legal fees and other expenses, diversion of management time, and disruption in our business. Uncertainties resulting from initiation and continuation of any patent or related litigation could harm our ability to compete.
We may not be able to enforce our intellectual property rights throughout the world.
The laws of some foreign countries, including India, where we manufacture pharmaceutical intermediates and APIs through contract manufacturers, do not protect intellectual property rights to the same extent as the laws of the United States. Many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biotechnology and/or bioindustrials technologies. This could make it difficult for us to stop the infringement of our patents or misappropriation of our other intellectual property rights. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business. Accordingly, our efforts to protect our intellectual property rights in such countries may be inadequate.
If our biocatalysts, or the genes that code for our biocatalysts, are stolen, misappropriated or reverse engineered, others could use these biocatalysts or genes to produce competing products.
Third parties, including our contract manufacturers, customers and those involved in shipping our biocatalysts often have custody or control of our biocatalysts. If our biocatalysts, or the genes that code for our biocatalysts, were stolen, misappropriated or reverse engineered, they could be used by other parties who may be able to reproduce these biocatalysts for their own commercial gain. If this were to occur, it would be difficult for us to challenge this type of use, especially in countries with limited intellectual property protection.
29
Table of Contents
Confidentiality agreements with employees and others may not adequately prevent disclosures of trade secrets and other proprietary information.
We rely in part on trade secret protection to protect our confidential and proprietary information and processes. However, trade secrets are difficult to protect. We have taken measures to protect our trade secrets and proprietary information, but these measures may not be effective. We require new employees and consultants to execute confidentiality agreements upon the commencement of an employment or consulting arrangement with us. These agreements generally require that all confidential information developed by the individual or made known to the individual by us during the course of the individuals relationship with us be kept confidential and not disclosed to third parties. These agreements also generally provide that inventions conceived by the individual in the course of rendering services to us shall be our exclusive property. Nevertheless, our proprietary information may be disclosed, third parties could reverse engineer our biocatalysts and others may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
Competitors and potential competitors who have greater resources and experience than we do may develop products and technologies that make ours obsolete or may use their greater resources to gain market share at our expense.
The biocatalysis industry and each of our target markets are characterized by rapid technological change. Our future success will depend on our ability to maintain a competitive position with respect to technological advances. We are aware that other companies, including Verenium Corporation (formed by the merger of Diversa Corporation and Celunol Corporation), Royal DSM N.V., or DSM, Danisco/Genencor, Novozymes and E.I. Du Pont De Nemours and Company, or DuPont, have alternative methods for obtaining and generating genetic diversity or use mutagenesis techniques to produce genetic diversity. Academic institutions such as the California Institute of Technology, the Max Planck Institute and the Center for Fundamental and Applied Molecular Evolution (FAME), a jointly sponsored initiative between Emory University and Georgia Institute of Technology, are also working in this field. Technological development by others may result in our products and technologies, as well as products developed by our customers using our biocatalysts, becoming obsolete.
We face intense competition in the pharmaceuticals market. There are a number of companies who compete with us throughout the various stages of a pharmaceutical products lifecycle. Many large pharmaceutical companies have internal capabilities to develop and manufacture intermediates and APIs. These companies include many of our large innovator and generic pharmaceutical customers, such as Merck, Pfizer and Teva Pharmaceutical Industries Ltd. There are also many large, well-established fine chemical manufacturing companies, such as DSM, BASF Corporation and Lonza Group Ltd, that compete to supply pharmaceutical intermediates and APIs to our customers. We also face increasing competition from generic pharmaceutical manufacturers in low cost centers such as India and China.
In addition to competition from companies manufacturing APIs and intermediates, we face competition from companies that sell biocatalysts for use in the pharmaceutical market. There is competition from large industrial enzyme companies, such as Novozymes and Amano Enzyme Inc., whose industrial enzymes (for detergents, for example) are occasionally used in pharmaceutical processes. There is also competition in this area from several small companies with product offerings comprised primarily of naturally occurring biocatalysts or that offer biocatalyst optimization services.
We expect the biofuels industry to be extremely competitive, with competition coming from ethanol producers as well as other providers of alternative and renewable fuels. Significant competitors include companies such as: Novozymes, which has partnered with a number of companies and organizations on a regional basis to develop or produce biofuels, and recently opened a biofuel demonstration plant with
30
Table of Contents
Inbicon A/S of Denmark; Danisco/Genencor, which has formed a joint venture with DuPont, called DuPont Danisco Cellulosic Ethanol, or DDCE, and is marketing a line of cellulases to convert biomass into sugar; DSM, which received a grant from the U.S. Department of Energy to be the lead partner in a technical consortium including Abengoa Bioenergy New Technologies, and is developing cost-effective enzyme technologies; Mascoma Corporation, which has entered into a feedstock processing and lignin supply agreement with Chevron Technology Ventures, a division of Chevron U.S.A., Inc.; and Verenium, which has entered into a research and development collaboration with BP, p.l.c and formed a joint venture with BP called Vercipia Biofuels to develop a commercial scale cellulosic ethanol facility. In addition, other companies are attempting to develop non-ethanol biofuels. DuPont has announced plans to develop and market biobutanol through Butamax Advanced Biofuels LLC, a joint venture with BP, and Virent Energy Systems Inc. is collaborating with Shell to develop thermochemical catalytic routes to produce biogasoline directly from sugars. Range Fuels Inc. is also focused on developing non-biocatalytic thermochemical processes to convert cellulosic biomass into fuels, and Coskata, Inc. is developing a hybrid thermochemical-biocatalytic process to produce ethanol from a variety of feedstocks. Some or all of these competitors or other competitors, as well as academic, research and government institutions, are developing or may develop technologies for, and are competing or may compete with us in, the production of alternative fuels or biofuels.
As we pursue opportunities in other bioindustrial markets, we expect to face competition from numerous companies focusing on developing biocatalytic and other solutions for these markets, including a number of the companies described above.
Our ability to compete successfully will depend on our ability to develop proprietary products that reach the market in a timely manner and are technologically superior to and/or are less expensive than other products on the market. Many of our competitors have substantially greater production, financial, research and development, personnel and marketing resources than we do. In addition, certain of our competitors may also benefit from local government subsidies and other incentives that are not available to us. As a result, our competitors may be able to develop competing and/or superior technologies and processes, and compete more aggressively and sustain that competition over a longer period of time than we could. Our technologies and products may be rendered obsolete or uneconomical by technological advances or entirely different approaches developed by one or more of our competitors. As more companies develop new intellectual property in our markets, the possibility of a competitor acquiring patent or other rights that may limit our products or potential products increases, which could lead to litigation.
In addition, various governments have recently announced a number of spending programs focused on the development of clean technology, including alternatives to petroleum-based fuels and the reduction of carbon emissions, two of our target markets. Such spending programs could lead to increased funding for our competitors or the rapid increase in the number of competitors within those markets.
Our limited resources relative to many of our competitors may cause us to fail to anticipate or respond adequately to new developments and other competitive pressures. This failure could reduce our competitiveness and market share, adversely affect our results of operations and financial position, and prevent us from obtaining or maintaining profitability.
We may need substantial additional capital in the future in order to expand our business.
Our future capital requirements may be substantial, particularly as we continue to develop our business and expand our biocatalyst discovery and development process. Although we believe that, based on our current level of operations and anticipated growth, our existing cash, cash equivalents and marketable securities will provide adequate funds for ongoing operations, planned capital expenditures and working capital requirements for at least the next 12 months, we may need additional capital if our current plans and assumptions change. Our need for additional capital will depend on many factors, including the
31
Table of Contents
financial success of our pharmaceutical business, whether we are successful in obtaining payments from customers, whether we can enter into additional collaborations, the progress and scope of our collaborative and independent research and development projects performed by us and our collaborators, the effect of any acquisitions of other businesses or technologies that we may make in the future, whether we decide to develop an internal manufacturing capability, and the filing, prosecution and enforcement of patent claims.
If our capital resources are insufficient to meet our capital requirements, and we are unable to enter into or maintain collaborations with partners that are able or willing to fund our development efforts or commercialize any products that we develop or enable, we will have to raise additional funds to continue the development of our technology and products and complete the commercialization of products, if any, resulting from our technologies. If future financings involve the issuance of equity securities, our existing stockholders would suffer dilution. If we were permitted to raise additional debt financing, we may be subject to restrictive covenants that limit our ability to conduct our business. We may not be able to raise sufficient additional funds on terms that are favorable to us, if at all. If we fail to raise sufficient funds and continue to incur losses, our ability to fund our operations, take advantage of strategic opportunities, develop products or technologies, or otherwise respond to competitive pressures could be significantly limited. If this happens, we may be forced to delay or terminate research or development programs or the commercialization of products resulting from our technologies, curtail or cease operations or obtain funds through collaborative and licensing arrangements that may require us to relinquish commercial rights, or grant licenses on terms that are not favorable to us. If adequate funds are not available, we will not be able to successfully execute our business plan or continue our business.
The terms of our loan and security agreement with General Electric Capital Corporation and Oxford Finance Corporation may restrict our ability to engage in certain transactions.
In September 2007, we entered into a loan and security agreement with General Electric Capital Corporation, or GE Capital, and Oxford Finance Corporation, or Oxford. Pursuant to the terms of the loan and security agreement, we cannot engage in certain transactions, including disposing of certain assets, transferring capital to foreign subsidiaries, incurring additional indebtedness, declaring dividends, acquiring or merging with another entity or leasing additional real property unless certain conditions are met or unless we receive prior approval of GE Capital and Oxford. If GE Capital and Oxford do not consent to any of these actions that we desire to take, we could be prohibited from engaging in transactions which could be beneficial to our business and our stockholders.
Business interruptions could delay us in the process of developing our products and could disrupt our sales.
Our headquarters is located in the San Francisco Bay Area near known earthquake fault zones and is vulnerable to significant damage from earthquakes. We are also vulnerable to other types of natural disasters and other events that could disrupt our operations, such as riot, civil disturbances, war, terrorist acts, flood, infections in our laboratory or production facilities or those of our contract manufacturers and other events beyond our control. We do not have a detailed disaster recovery plan. In addition, we do not carry insurance for earthquakes and we may not carry sufficient business interruption insurance to compensate us for losses that may occur. Any losses or damages we incur could have a material adverse effect on our cash flows and success as an overall business. Furthermore, Shell may terminate our collaborative research agreement if a force majeure event interrupts our collaboration activities for more than ninety days.
Ethical, legal and social concerns about genetically engineered products and processes could limit or prevent the use of our products, processes, and technologies and limit our revenues.
Some of our products and processes are genetically engineered or involve the use of genetically engineered products or genetic engineering technologies. If we and/or our collaborators are not able to
32
Table of Contents
overcome the ethical, legal, and social concerns relating to genetic engineering, our products and processes may not be accepted. Any of the risks discussed below could result in increased expenses, delays, or other impediments to our programs or the public acceptance and commercialization of products and processes dependent on our technologies or inventions. Our ability to develop and commercialize one or more of our technologies, products, or processes could be limited by the following factors:
| | public attitudes about the safety and environmental hazards of, and ethical concerns over, genetic research and genetically engineered products and processes, which could influence public acceptance of our technologies, products and processes; |
| | public attitudes regarding, and potential changes to laws governing ownership of genetic material, which could harm our intellectual property rights with respect to our genetic material and discourage collaborators from supporting, developing, or commercializing our products, processes and technologies; and |
| | governmental reaction to negative publicity concerning genetically modified organisms, which could result in greater government regulation of genetic research and derivative products. |
The subject of genetically modified organisms has received negative publicity, which has aroused public debate. This adverse publicity could lead to greater regulation and trade restrictions on imports of genetically altered products.
The biocatalysts that we develop have significantly enhanced characteristics compared to those found in naturally occurring enzymes or microbes. While we produce our biocatalysts only for use in a controlled industrial environment, the release of such biocatalysts into uncontrolled environments could have unintended consequences. Any adverse effect resulting from such a release could have a material adverse effect on our business and financial condition, and we may have exposure to liability for any resulting harm.
Compliance with stringent laws and regulations may be time consuming and costly, which could adversely affect the commercialization of our biofuels products.
Any biofuels developed using our technologies will need to meet a significant number of regulations and standards, including regulations imposed by the U.S. Department of Transportation, the U.S. Environmental Protection Agency, various state agencies and others. Any failure to comply, or delays in compliance, with the various existing and evolving industry regulations and standards could prevent or delay the commercialization of any biofuels developed using our technologies and subject us to fines and other penalties.
We use hazardous materials in our business and we must comply with environmental laws and regulations. Any claims relating to improper handling, storage or disposal of these materials or noncompliance of applicable laws and regulations could be time consuming and costly and could adversely affect our business and results of operations.
Our research and development processes involve the use of hazardous materials, including chemical, radioactive, and biological materials. Our operations also produce hazardous waste. We cannot eliminate entirely the risk of accidental contamination or discharge and any resultant injury from these materials. Federal, state, and local laws and regulations govern the use, manufacture, storage, handling and disposal of, and human exposure to, these materials. We may be sued for any injury or contamination that results from our use or the use by third parties of these materials, and our liability may exceed our total assets. Although we believe that our activities conform in all material respects with environmental laws, there can be no assurance that violations of environmental, health and safety laws will not occur in the future as a result of human error, accident, equipment failure or other causes. Compliance with applicable environmental laws and regulations may be expensive, and the failure to comply with past, present, or
33
Table of Contents
future laws could result in the imposition of fines, third party property damage, product liability and personal injury claims, investigation and remediation costs, the suspension of production, or a cessation of operations, and our liability may exceed our total assets. Liability under environmental laws can be joint and several and without regard to comparative fault. Environmental laws could become more stringent over time imposing greater compliance costs and increasing risks and penalties associated with violations, which could impair our research, development or production efforts and harm our business.
We may be sued for product liability.
The design, development, manufacture and sale of our products involve an inherent risk of product liability claims and the associated adverse publicity. We may be named directly in product liability suits relating to drugs that are produced using our biocatalysts or that incorporate our intermediates and APIs. These claims could be brought by various parties, including customers who are purchasing products directly from us, other companies who purchase products from our customers or by the end users of the drugs. We could also be named as co-parties in product liability suits that are brought against our contract manufacturers who manufacture our pharmaceutical intermediates and APIs, such as Arch. Insurance coverage is expensive and may be difficult to obtain, and may not be available in the future on acceptable terms, or at all. We cannot assure you that our contract manufacturers will have adequate insurance coverage to cover against potential claims. In addition, although we currently maintain product liability insurance for our products in amounts we believe to be commercially reasonable, if the coverage limits of these insurance policies are not adequate, a claim brought against us, whether covered by insurance or not, could have a material adverse effect on our business, results of operations, financial condition and cash flows. This insurance may not provide adequate coverage against potential losses, and if claims or losses exceed our liability insurance coverage, we may go out of business. Moreover, we have agreed to indemnify some of our customers for certain claims that may arise out of the use of our products, which could expose us to significant liabilities.
Our ability to use our net operating loss carryforwards to offset future taxable income may be subject to certain limitations.
In general, under Section 382 of the Internal Revenue Code, a corporation that undergoes an ownership change is subject to limitations on its ability to utilize its pre-change net operating loss carryforwards, or NOLs, to offset future taxable income. If the Internal Revenue Service challenges our analysis that our existing NOLs are not subject to limitations arising from previous ownership changes, or if we undergo an ownership change in connection with or after this public offering, our ability to utilize NOLs could be limited by Section 382 of the Internal Revenue Code. Future changes in our stock ownership, some of which are outside of our control, could result in an ownership change under Section 382 of the Internal Revenue Code. Furthermore, our ability to utilize NOLs of companies that we may acquire in the future may be subject to limitations. For these reasons, we may not be able to utilize a material portion of the NOLs reflected on our balance sheet, even if we attain profitability.
Risks Relating to this Offering
We are subject to anti-takeover provisions in our certificate of incorporation and bylaws and under Delaware law that could delay or prevent an acquisition of our company, even if the acquisition would be beneficial to our stockholders.
Provisions in our amended and restated certificate of incorporation and our bylaws, both of which will become effective upon the completion of this offering, may delay or prevent an acquisition of us. Among other things, our amended and restated certificate of incorporation and bylaws will provide for a board of directors which is divided into three classes, with staggered three-year terms and will provide that all
34
Table of Contents
stockholder action must be effected at a duly called meeting of the stockholders and not by a consent in writing, and will further provide that only our board of directors, the chairman of the board of directors, our chief executive officers or president may call a special meeting of the stockholders. These provisions may also frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, who are responsible for appointing the members of our management team. Furthermore, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which prohibits, with some exceptions, stockholders owning in excess of 15% of our outstanding voting stock from merging or combining with us. Finally, our charter documents establish advanced notice requirements for nominations for election to our board of directors and for proposing matters that can be acted upon at stockholder meetings. Although we believe these provisions together provide for an opportunity to receive higher bids by requiring potential acquirers to negotiate with our board of directors, they would apply even if an offer to acquire our company may be considered beneficial by some stockholders.
Concentration of ownership among our existing officers, directors and principal stockholders may prevent other stockholders from influencing significant corporate decisions and depress our stock price.
When this offering is completed, our officers, directors and existing stockholders who hold at least 5% of our stock will together control approximately % of our outstanding common stock. As of September 30, 2009, Maxygen, Shell and Biomedical Sciences Investment Fund Pte Ltd beneficially owned approximately 21.6%, 20% and 12.1% of our common stock, respectively. If these officers, directors, and principal stockholders or a group of our principal stockholders act together, they will be able to exert a significant degree of influence over our management and affairs and control matters requiring stockholder approval, including the election of directors and approval of mergers or other business combination transactions. The interests of this concentration of ownership may not always coincide with our interests or the interests of other stockholders. For instance, officers, directors, and principal stockholders, acting together, could cause us to enter into transactions or agreements that we would not otherwise consider. Similarly, this concentration of ownership may have the effect of delaying or preventing a change in control of our company otherwise favored by our other stockholders. This concentration of ownership could depress our stock price.
Our share price may be volatile and you may be unable to sell your shares at or above the offering price.
The initial public offering price for our shares will be determined by negotiations between us and representatives of the underwriters and may not be indicative of prices that will prevail in the trading market. The market price of shares of our common stock could be subject to wide fluctuations in response to many risk factors listed in this section, and others beyond our control, including:
| | actual or anticipated fluctuations in our financial condition and operating results; |
| | the position of our cash, cash equivalents and marketable securities; |
| | actual or anticipated changes in our growth rate relative to our competitors; |
| | actual or anticipated fluctuations in our competitors operating results or changes in their growth rate; |
| | announcements of technological innovations by us, our collaborators or our competitors; |
| | announcements by us, our collaborators or our competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments; |
| | any changes in Shells biofuels strategy or timelines, or in our relationship with Shell, including any decision by Shell to terminate our collaboration or reduce the number of FTEs funded by Shell under our collaborative research agreement; |
35
Table of Contents
| | any changes in our relationship with Maxygen, or any events that impact, or are perceived to impact, the rights we have licensed from Maxygen; |
| | announcements regarding pharmaceutical products manufactured using our biocatalysts, intermediates and APIs; |
| | the entry into, modification or termination of collaborative arrangements; |
| | additions or losses of customers; |
| | additions or departures of key management or scientific personnel; |
| | competition from existing products or new products that may emerge; |
| | issuance of new or updated research reports by securities or industry analysts; |
| | fluctuations in the valuation of companies perceived by investors to be comparable to us; |
| | disputes or other developments related to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies; |
| | changes in existing laws, regulations and policies applicable to our business and products, including the National Renewable Fuel Standard program, and the adoption or failure to adopt carbon emissions regulation; |
| | announcement or expectation of additional financing efforts; |
| | sales of our common stock by us or our stockholders; |
| | share price and volume fluctuations attributable to inconsistent trading volume levels of our shares; |
| | general market conditions in our industry; and |
| | general economic and market conditions, including the recent financial crisis. |
Furthermore, the stock markets have experienced extreme price and volume fluctuations that have affected and continue to affect the market prices of equity securities of many companies. These fluctuations often have been unrelated or disproportionate to the operating performance of those companies. These broad market and industry fluctuations, as well as general economic, political and market conditions such as recessions, interest rate changes or international currency fluctuations, may negatively impact the market price of shares of our common stock. If the market price of shares of our common stock after this offering does not exceed the initial public offering price, you may not realize any return on your investment in us and may lose some or all of your investment. In the past, companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation against us could result in substantial costs and divert our managements attention from other business concerns, which could seriously harm our business.
A significant portion of our total outstanding shares of common stock is restricted from immediate resale but may be sold into the market in the near future. This could cause the market price of our common stock to drop significantly, even if our business is doing well.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares of common stock intend to sell shares, could reduce the market price of our common stock. As of September 30, 2009, our three largest stockholders beneficially own, collectively, approximately 54% of our outstanding common stock. If one or more of them were to sell a substantial portion of the shares they hold, it could cause our stock price to decline. Based on shares outstanding as of September 30, 2009, upon completion of this offering, we will have outstanding shares of common stock, assuming no exercise of the
36
Table of Contents
underwriters option to purchase additional shares. This includes the shares that we and the selling stockholders are selling in this offering. Of the remaining shares, shares of common stock will be subject to a 180-day contractual lock-up with the underwriters, and shares of common stock will be subject to a 180-day contractual lock-up with us.
In addition, as of September 30, 2009, there were shares subject to outstanding options that will become eligible for sale in the public market to the extent permitted by any applicable vesting requirements, the lock-up agreements and Rules 144 and 701 under the Securities Act of 1933, as amended. Moreover, after this offering, holders of an aggregate of approximately shares of our common stock, will have rights, subject to some conditions, to require us to file registration statements covering their shares and to include their shares in registration statements that we may file for ourselves or other stockholders. Holders of an aggregate of approximately additional shares of our common stock as of September 30, 2009, will have rights, subject to some conditions, to include their shares in registration statements that we may file for ourselves or other stockholders.
We also intend to register all shares of common stock that we may issue under our 2010 Equity Incentive Award Plan. Once we register these shares, they can be freely sold in the public market upon issuance and once vested, subject to the 180-day lock-up periods under the lock-up agreements described in the Underwriting section of this prospectus.
No public market for our common stock currently exists and an active trading market may not develop or be sustained following this offering.
Prior to this offering, there has been no public market for our common stock. An active trading market may not develop following the completion of this offering or, if developed, may not be sustained. The lack of an active market may impair your ability to sell your shares at the time you wish to sell them or at a price that you consider reasonable. The lack of an active market may also reduce the fair market value of your shares. An inactive market may also impair our ability to raise capital to continue to fund operations by selling shares and may impair our ability to acquire other companies or technologies by using our shares as consideration.
If securities or industry analysts do not publish research or reports about our business, or publish negative reports about our business, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that securities or industry analysts publish about us or our business. We do not have any control over these analysts. If one or more of the analysts who cover us downgrade our stock or change their opinion of our stock, our stock price would likely decline. If one or more of these analysts cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause our stock price or trading volume to decline.
Purchasers in this offering will experience immediate and substantial dilution in the book value of their investment.
The initial public offering price will be substantially higher than the tangible book value per share of shares of our common stock based on the total value of our tangible assets less our total liabilities immediately following this offering. Therefore, if you purchase shares of our common stock in this offering, you will experience immediate and substantial dilution of approximately $ per share in the price you pay for shares of our common stock as compared to its tangible book value, assuming an initial public offering price of $ per share. To the extent outstanding options and warrants to purchase shares of common stock are exercised, there will be further dilution. For further information on this calculation, see Dilution elsewhere in this prospectus.
37
Table of Contents
We have broad discretion in the use of net proceeds from this offering and may not use them effectively.
Although we currently intend to use the net proceeds from this offering in the manner described in Use of Proceeds elsewhere in this prospectus, we will have broad discretion in the application of the net proceeds. Our failure to apply these net proceeds effectively could affect our ability to continue to develop and sell our products and grow our business, which could cause the value of your investment to decline.
We will incur significant increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives.
We have never operated as a stand-alone public company. As a public company, we will incur significant legal, accounting and other expenses that we did not incur as a private company. In addition, the Sarbanes-Oxley Act, as well as related rules implemented by the Securities and Exchange Commission and The Nasdaq Stock Market, imposes various requirements on public companies. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly. For example, we expect these rules and regulations to make it more expensive for us to maintain director and officer liability insurance.
In addition, the Sarbanes-Oxley Act requires, among other things, that we maintain effective internal control over financial reporting and disclosure controls and procedures. In particular, commencing in 2011, we must perform system and process evaluation and testing of our internal control over financial reporting to allow management and our independent registered public accounting firm to report on the effectiveness of our internal control over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act. We may not be able to remediate the material weakness in our internal control over financial reporting prior to the time of this testing. Our compliance with Section 404 will require that we incur substantial accounting expense and expend significant management time on compliance-related issues. We will need to hire additional accounting and financial staff with appropriate public company experience and technical accounting knowledge. Moreover, if we are not able to comply with the requirements of Section 404 in a timely manner, or if we are unable to remediate the material weakness in our internal control over financial reporting in a timely manner, our stock price could decline, and we could face sanctions, delisting or investigations by The Nasdaq Global Market, or other material effects on our business, reputation, results of operations, financial condition or liquidity.
We do not anticipate paying cash dividends, and accordingly, stockholders must rely on stock appreciation for any return on their investment.
The terms of our loan and security agreement with GE Capital and Oxford currently prohibit us from paying cash dividends on our common stock. In addition, we do not anticipate paying cash dividends in the future. As a result, only appreciation of the price of our common stock, which may never occur, will provide a return to stockholders. Investors seeking cash dividends should not invest in our common stock.
38
Table of Contents
This prospectus contains forward-looking statements that involve risks and uncertainties. The forward-looking statements are contained principally in the sections entitled Prospectus Summary, Risk Factors, Managements Discussion and Analysis of Financial Condition and Results of Operations and Business. These statements relate to future events or our future financial or operational performance and involve known and unknown risks, uncertainties and other factors that could cause our actual results, levels of activity, performance or achievement to differ materially from those expressed or implied by these forward-looking statements. These risks and uncertainties are contained principally in the section entitled Risk Factors.
Forward-looking statements include all statements that are not historical facts. In some cases, you can identify forward-looking statements by terms such as may, will, should, could, would, expects, plans, anticipates, believes, estimates, projects, predicts, potential, or the negative of those terms, and similar expressions and comparable terminology intended to identify forward-looking statements. These statements reflect our current views with respect to future events and are based on assumptions and subject to risks and uncertainties. Given these uncertainties, you should not place undue reliance on these forward-looking statements. These forward-looking statements represent our estimates and assumptions only as of the date of this prospectus and, except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise after the date of this prospectus.
This prospectus also contains estimates and other information concerning our current and target markets that are based on industry publications, surveys and forecasts, including those generated by IMS Health, Datamonitor and the U.S. Energy Information Administration. This information involves a number of assumptions and limitations, and you are cautioned not to give undue weight to these estimates and information. These industry publications, surveys and forecasts generally indicate that their information has been obtained from sources believed to be reliable. The industry in which we operate is subject to a high degree of uncertainty and risk due to a variety of factors, including those described in Risk Factors. These and other factors could cause actual results to differ materially from those expressed in these publications, surveys and forecasts.
39
Table of Contents
We estimate that we will receive net proceeds of approximately $ million from the sale of shares of common stock offered in this offering and the selling stockholders will receive net proceeds of approximately $ million from the sale of shares of common stock offered in this offering, based on an assumed initial public offering price of $ per share (the mid-point of the price range set forth on the cover page of this prospectus) and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us and the selling stockholders. A $1.00 increase (decrease) in the assumed initial public offering price of $ per share would increase (decrease) the net proceeds to us from this offering by $ million and increase (decrease) the net proceeds to the selling stockholders by $ million, assuming that the number of shares offered by us and the selling stockholders, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us and the selling stockholders. If the underwriters exercise their option to purchase additional shares in full, we estimate that our net proceeds will be approximately $ million.
We intend to use the net proceeds of this offering, together with existing cash and cash equivalents, to fund working capital and other general corporate purposes, including the costs associated with being a public company. We may also use a portion of the net proceeds to acquire other businesses, products or technologies, and to increase our internal biocatalyst production capacity. We do not have agreements or commitments for any specific acquisitions at this time.
The expected use of net proceeds of this offering represents our current intentions based upon our present plan and business conditions. As of the date of this prospectus, we cannot specify with certainty all of the particular uses for the net proceeds to be received upon the completion of this offering. Accordingly, we will have broad discretion in the application of the net proceeds, and investors will be relying on our judgment regarding the application of the net proceeds of this offering.
Until we use the net proceeds of this offering, we intend to invest the net proceeds in short-term, interest-bearing, investment-grade securities. We cannot predict whether the net proceeds invested will yield a favorable return.
We have never declared or paid cash dividends on our common stock, and currently do not plan to declare dividends on shares of our common stock in the foreseeable future. In addition, the terms of our loan and security agreement with General Electric Capital Corporation and Oxford Finance Corporation currently prohibit us from paying cash dividends on our common stock. We expect to retain our future earnings, if any, for use in the operation and expansion of our business. In addition, in certain circumstances, we are prohibited by various borrowing arrangements from paying cash dividends without the prior written consent of the lenders. Subject to the foregoing, the payment of cash dividends in the future, if any, will be at the discretion of our board of directors and will depend upon such factors as earnings levels, capital requirements, our overall financial condition and any other factors deemed relevant by our board of directors.
40
Table of Contents
The following table sets forth our cash, cash equivalents and marketable securities and our capitalization as of September 30, 2009:
| | on an actual basis; |
| | on a pro forma basis to reflect: |
| | the filing of a restated certificate of incorporation to authorize shares of common stock and shares of undesignated preferred stock; |
| | the conversion of all of our outstanding shares of redeemable convertible preferred stock into 37,624,216 shares of common stock and the related conversion of all outstanding redeemable convertible preferred stock warrants to common stock warrants; |
| | the reclassification of the redeemable convertible preferred stock warrant liability to stockholders equity upon the completion of this offering; and |
| | on a pro forma as adjusted basis to reflect the pro forma adjustments described above and our receipt of the estimated net proceeds from this offering, based on an assumed initial public offering of shares at a price of $ per share (the mid-point of the price range set forth on the cover page of this prospectus) and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
The pro forma and pro forma as adjusted information below is illustrative only and our capitalization following the completion of this offering will be adjusted based on the actual initial public offering price and other terms of this offering determined at pricing. You should read this table together with Managements Discussion and Analysis of Financial Condition and Results of Operations and our consolidated financial statements and the accompanying notes appearing elsewhere in this prospectus.
| As of September 30, 2009 | |||||||||||
| Actual | Pro Forma | Pro Forma As Adjusted |
|||||||||
| (unaudited) | |||||||||||
| (in thousands, except share data) | |||||||||||
| Cash, cash equivalents and marketable securities |
$ | 55,962 | $ | 55,962 | $ | ||||||
| Redeemable convertible preferred stock warrant liability |
$ | 1,731 | $ | | $ | ||||||
| Financing obligations, net of current portion |
3,412 | 3,412 | |||||||||
| Redeemable convertible preferred stock, $0.0001 par value per share; 39,204,886 shares authorized, 37,563,610 shares issued and outstanding, actual; no shares authorized, no shares issued and outstanding, pro forma and pro forma as adjusted |
177,746 | | |||||||||
| Stockholders equity (deficit): |
|||||||||||
| Preferred stock, $0.0001 par value per share; no shares authorized, issued and outstanding, actual; shares authorized, no shares issued and outstanding, pro forma; shares authorized, no shares issued and outstanding, pro forma as adjusted |
| ||||||||||
| Common stock, $0.0001 par value per share; 68,000,000 shares authorized; 3,975,552 issued and outstanding, actual; shares authorized, 41,599,768 shares issued and outstanding, pro forma; shares authorized, shares issued and outstanding, pro forma as adjusted |
| 4 | |||||||||
| Additional paid-in capital |
13,324 | 192,797 | |||||||||
| Accumulated other comprehensive income |
211 | 211 | |||||||||
| Accumulated deficit |
(154,428 | ) | (154,428 | ) | |||||||
| Total stockholders equity (deficit) |
(140,893 | ) | 38,584 | ||||||||
| Total capitalization |
$ | 41,996 | $ | 41,996 | $ | ||||||
41
Table of Contents
Each $1.00 increase or decrease in the assumed initial public offering price of $ per share (the mid-point of the price range set forth on the cover page of this prospectus) would increase or decrease, as applicable, our pro forma as adjusted cash, cash equivalents and marketable securities, additional paid-in capital and stockholders equity by approximately $ million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
The number of shares of common stock shown as issued and outstanding in the table is based on the number of shares of our common stock outstanding as of September 30, 2009 and excludes:
| | 10,962,854 shares of common stock issuable upon the exercise of options outstanding as of September 30, 2009 at a weighted average exercise price of $3.25 per share; |
| | 491,513 shares of common stock issuable upon the exercise of warrants outstanding as of September 30, 2009 at a weighted average exercise price of $3.95 per share; and |
| | shares of our common stock reserved for future issuance under our 2010 Equity Incentive Award Plan, which will become effective in connection with the consummation of this offering (including 3,226,648 shares of common stock reserved for future grant or issuance under our 2002 Stock Plan as of September 30, 2009, which shares will be added to the shares to be reserved under our 2010 Equity Incentive Award Plan upon the effectiveness of the 2010 Equity Incentive Award Plan). |
42
Table of Contents
If you invest in our common stock, your interest will be diluted to the extent of the difference between the public offering price per share of our common stock and the pro forma as adjusted net tangible book value per share of our common stock after this offering.
Our pro forma net tangible book value at September 30, 2009 was $34.2 million, or $0.82 per share of common stock. Pro forma net tangible book value per share represents total tangible assets less total liabilities (which includes the reclassification of redeemable convertible preferred stock warrant liability into additional paid-in capital upon the conversion of outstanding shares of preferred stock underlying warrants into shares of common stock), divided by the number of outstanding shares of common stock on September 30, 2009, after giving effect to the conversion of all outstanding shares of preferred stock into shares of common stock as if the conversion occurred on September 30, 2009. Our pro forma as adjusted net tangible book value at September 30, 2009, after giving effect to the sale by us of shares of common stock in this offering at an assumed initial public offering price of $ per share (the mid-point of the price range set forth on the cover page of this prospectus) and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, would have been approximately $ million, or $ per share. This represents an immediate increase in pro forma as adjusted net tangible book value of $ per share to existing stockholders and an immediate dilution of $ per share to new investors, or approximately % of the assumed initial public offering price of $ per share. The following table illustrates this per share dilution:
| Assumed initial public offering price per share |
$ | |||||
| Pro forma net tangible book value per share at September 30, 2009 |
$ | 0.82 | ||||
| Increase in pro forma net tangible book value per share attributable to this offering |
||||||
| Pro forma as adjusted net tangible book value per share after this offering |
||||||
| Dilution per share to new investors |
$ | |||||
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share (the mid-point of the price range set forth on the cover page of this prospectus) would increase (decrease) our pro forma as adjusted net tangible book value by $ million, the pro forma as adjusted net tangible book value per share by $ per share and the dilution in the pro forma net tangible book value to new investors in this offering by $ per share, assuming the number of shares offered by us, as set forth on the cover pages of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
The following table shows, as of September 30, 2009, the number of shares of common stock purchased from us, the total consideration paid to us and the average price paid per share by existing stockholders and by new investors purchasing common stock in this offering at an assumed initial public offering price of $ per share, before deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
| Shares Purchased | Total Consideration |
Average Price Per Share |
||||||||||||
| Number | Percent | Amount | Percent | |||||||||||
| Existing stockholders |
% | $ | % | $ | ||||||||||
| New investors |
||||||||||||||
| Total |
100.0 | % | $ | 100.0 | % | $ | ||||||||
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share (the mid-point of the price range set forth on the cover page of this prospectus) would increase (decrease) total consideration paid by new investors, total consideration paid by all stockholders and the average price per
43
Table of Contents
share paid by all stockholders by $ , $ and $ , respectively, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the underwriting discount and estimated offering expenses payable by us.
The sale of shares of our common stock to be sold by the selling stockholders in this offering will reduce the number of shares of our common stock held by existing stockholders to , or % of the total shares outstanding, and will increase the number of shares of our common stock held by new investors to , or % of the total shares of our common stock outstanding.
The discussion and tables in this section regarding dilution are based on 41,599,768 shares of common stock issued and outstanding as of September 30, 2009 which reflects the automatic conversion of all of our preferred stock into an aggregate of 37,624,216 shares of our common stock, and excludes:
| | shares of common stock issuable upon the exercise of 10,962,854 options outstanding at a weighted average exercise price of $3.25 per share; |
| | shares of common stock issuable upon exercise of 491,513 warrants outstanding at a weighted average exercise price of $3.95 per share; and |
| | shares of common stock reserved for issuance under our 2010 Equity Incentive Award Plan, which will become effective upon the completion of this offering (plus an additional 3,226,648 shares of common stock reserved for future grant or issuance under our 2002 Stock Plan as of September 30, 2009, which shares will be added to the shares to be reserved under our 2010 Equity Incentive Award Plan upon the effectiveness of the 2010 Equity Incentive Award Plan). |
If the underwriters exercise their option to purchase additional shares in full, the following will occur:
| | the number of shares of our common stock held by existing stockholders would decrease to approximately % of the total number of shares of our common stock outstanding after this offering; and |
| | the number of shares of our common stock held by new investors would increase to approximately % of the total number of shares of our common stock outstanding after this offering. |
To the extent that outstanding options or warrants are exercised, you will experience further dilution. If all of our outstanding options and warrants were exercised, our pro forma net tangible book value as of September 30, 2009 would have been $71.8 million, or $1.35 per share, and the pro forma, as adjusted net tangible book value after this offering would have been $ million, or $ per share, causing dilution to new investors of $ per share.
In addition, we may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
44
Table of Contents
SELECTED CONSOLIDATED FINANCIAL DATA
The following selected consolidated financial data should be read together with our consolidated financial statements and accompanying notes and Managements Discussion and Analysis of Financial Condition and Results of Operations appearing elsewhere in this prospectus. The selected consolidated financial data in this section is not intended to replace our consolidated financial statements and the accompanying notes. Our historical results are not necessarily indicative of our future results.
We derived the consolidated statements of operations data for 2006, 2007 and 2008 and the consolidated balance sheets data as of December 31, 2007 and 2008 from our audited consolidated financial statements appearing elsewhere in this prospectus. The consolidated statement of operations data for 2004 and 2005 and the consolidated balance sheets data as of December 31, 2004, 2005 and 2006 have been derived from our audited consolidated financial statements not included in this prospectus. The consolidated statements of operations data for the nine months ended September 30, 2008 and 2009 and the consolidated balance sheet data as of September 30, 2009 are derived from our unaudited consolidated financial statements appearing elsewhere in this prospectus. The unaudited interim financial statements have been prepared on the same basis as the annual consolidated financial statements and, in the opinion of management, reflect all adjustments, which include only normal recurring adjustments, necessary to state fairly our financial position as of September 30, 2009 and results of operations and cash flows for the nine months ended September 30, 2008 and 2009. Operating results for the nine months ended September 30, 2009 are not necessarily indicative of the results that may be expected for the year ended December 31, 2009. The data should be read in conjunction with the consolidated financial statements, related notes, and other financial information included herein.
| Years Ended December 31, |
Nine Months Ended September 30, |
|||||||||||||||||||||||||||
| 2004 | 2005 | 2006 | 2007 | 2008 | 2008 | 2009 | ||||||||||||||||||||||
| (in thousands, except per share amounts) | ||||||||||||||||||||||||||||
| Consolidated Statements of Operations Data: |
||||||||||||||||||||||||||||
| Revenues: |
||||||||||||||||||||||||||||
| Product |
$ | | $ | 2,265 | $ | 2,544 | $ | 11,418 | $ | 16,860 | $ | 10,777 | $ | 13,401 | ||||||||||||||
| Related party collaborative research and development |
| | 863 | 8,481 | 30,239 | 18,174 | 43,963 | |||||||||||||||||||||
| Collaborative research and development |
4,873 | 9,363 | 8,403 | 4,733 | 3,062 | 2,678 | 1,295 | |||||||||||||||||||||
| Government grants |
| 156 | 317 | 701 | 317 | 251 | 11 | |||||||||||||||||||||
| Total revenues |
4,873 | 11,784 | 12,127 | 25,333 | 50,478 | 31,880 | 58,670 | |||||||||||||||||||||
| Costs and operating expenses: |
||||||||||||||||||||||||||||
| Cost of product revenues |
| 2,233 | 1,806 | 8,319 | 13,188 | 7,922 | 11,886 | |||||||||||||||||||||
| Research and development |
12,891 | 12,839 | 17,257 | 35,644 | 45,554 | 33,250 | 39,486 | |||||||||||||||||||||
| Selling, general and administrative |
5,187 | 7,891 | 11,880 | 19,713 | 35,709 | 28,300 | 20,939 | |||||||||||||||||||||
| Total costs and operating expenses |
18,078 | 22,963 | 30,943 | 63,676 | 94,451 | 69,472 | 72,311 | |||||||||||||||||||||
| Loss from operations |
(13,205 | ) | (11,179 | ) | (18,816 | ) | (38,343 | ) | (43,973 | ) | (37,592 | ) | (13,641 | ) | ||||||||||||||
| Interest income |
240 | 245 | 742 | 1,491 | 1,538 | 1,435 | 141 | |||||||||||||||||||||
| Interest expense and other, net |
(128 | ) | (413 | ) | (724 | ) | (2,533 | ) | (2,365 | ) | (2,517 | ) | (1,530 | ) | ||||||||||||||
| Loss before provision (benefit) for income taxes |
(13,093 | ) | (11,347 | ) | (18,798 | ) | (39,385 | ) | (44,800 | ) | (38,674 | ) | (15,030 | ) | ||||||||||||||
| Provision (benefit) for income taxes |
| 243 | (127 | ) | (408 | ) | 327 | 126 | 79 | |||||||||||||||||||
| Net loss |
(13,093 | ) | (11,590 | ) | (18,671 | ) | (38,977 | ) | (45,127 | ) | (38,800 | ) | (15,109 | ) | ||||||||||||||
| Accretion of redeemable convertible preferred stock(1) |
(1,250 | ) | | | | | | | ||||||||||||||||||||
| Net loss attributable to common stockholders |
$ | (14,343 | ) | $ | (11,590 | ) | $ | (18,671 | ) | $ | (38,977 | ) | $ | (45,127 | ) | $ | (38,800 | ) | $ | (15,109 | ) | |||||||
| Net loss attributable to common stockholders per share of common stock, basic and diluted(2) |
$ | (13.38 | ) | $ | (7.69 | ) | $ | (10.99 | ) | $ | (15.53 | ) | $ | (12.64 | ) | $ | (11.02 | ) | $ | (3.86 | ) | |||||||
| Weighted average common shares used in computing net loss per share of common stock, basic and diluted(2) |
1,072 | 1,508 | 1,699 | 2,510 | 3,570 | 3,521 | 3,917 | |||||||||||||||||||||
| Net loss used in computing pro forma net loss per share of common stock, basic and diluted (unaudited)(2) |
$ | (45,230 | ) | $ | (14,760 | ) | ||||||||||||||||||||||
| Pro forma net loss per share of common stock, basic and diluted (unaudited)(2) |
$ | (1.26 | ) | $ | (0.37 | ) | ||||||||||||||||||||||
| Weighted average common shares used in computing pro forma net loss per share of common stock, basic and diluted (unaudited)(2) |
35,900 | 39,684 | ||||||||||||||||||||||||||
45
Table of Contents
| (1) | During 2004, we recorded accretion to increase the redeemable convertible preferred stock to its redemption value due to the voting majority held by a certain stockholder which could effect a liquidation of the redeemable convertible preferred stock, pursuant to EITF Topic D-98 (incorporated in Accounting Standards Codification, or ASC, Topic 480, Distinguishing Liabilities from Equity). In 2005, the probability of the liquidation of the redeemable convertible preferred stock was reduced and accordingly we no longer recorded the related accretion subsequent to December 31, 2004. |
| (2) | Net loss used in computing pro forma basic and diluted net loss per share of common stock, pro forma basic and diluted net loss per share of common stock and the number of weighted average common shares used in computing the pro forma basic and diluted net loss per share of common stock in the table above give effect to the automatic conversion of all of our outstanding redeemable convertible preferred stock into common stock upon the closing of this offering as if such conversion had occurred at the beginning of each period or upon issuance, if later. |
| December 31, | September 30, | |||||||||||||||||||||||
| 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | |||||||||||||||||||
| (in thousands) | ||||||||||||||||||||||||
| Consolidated Balance Sheets Data: |
||||||||||||||||||||||||
| Cash, cash equivalents and marketable securities |
$ | 16,734 | $ | 7,005 | $ | 32,246 | $ | 84,070 | $ | 37,130 | $ | 55,962 | ||||||||||||
| Working capital |
12,837 | 2,781 | 22,972 | 60,732 | 5,933 | 28,061 | ||||||||||||||||||
| Total assets |
23,276 | 21,380 | 46,659 | 113,541 | 70,882 | 93,207 | ||||||||||||||||||
| Current and long-term financing obligations |
2,306 | 4,017 | 4,073 | 17,407 | 13,348 | 9,505 | ||||||||||||||||||
| Redeemable convertible preferred stock |
27,779 | 37,750 | 77,513 | 132,746 | 132,746 | 177,746 | ||||||||||||||||||
| Total stockholders deficit |
(12,984 | ) | (34,774 | ) | (52,766 | ) | (87,468 | ) | (129,124 | ) | (140,893 | ) | ||||||||||||
46
Table of Contents
MANAGEMENTS DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our consolidated financial statements and related notes that appear elsewhere in this prospectus. In addition to historical financial information, the following discussion contains forward-looking statements that involve risks and uncertainties. Our actual results may differ materially from those discussed below. Factors that could cause or contribute to these differences include those discussed below and elsewhere in this prospectus, particularly in Risk Factors.
Overview
Our proprietary technology platform enables the creation of optimized biocatalysts that make existing industrial processes faster, cleaner and more efficient than current methods and has the potential to make new industrial processes possible on a commercial scale. We have commercialized our biocatalysts in the pharmaceutical industry and are developing biocatalysts for use in producing advanced biofuels under a multi-year research and development collaboration with Shell. We are also using our technology platform to pursue biocatalyst-enabled solutions in other bioindustrial markets, including carbon management, water treatment and chemicals.
We were incorporated in Delaware in January 2002 as a wholly-owned subsidiary of Maxygen, Inc. In March 2002, we licensed from Maxygen core enabling technology and commenced operations. From 2002 until 2005, our operations focused on organizing and staffing our company and developing our technology platform. In 2005, we recognized our first revenues from product sales to the pharmaceutical industry. In 2006, we entered into our initial research and development collaboration with Equilon Enterprises LLC dba Shell Oil Products US, or Shell, an affiliate of Royal Dutch Shell plc, in the biofuels market.
To date, we have generated revenues primarily from collaborative research and development funding, pharmaceutical product sales and government grants. Over the last three fiscal years, revenues grew from $12.1 million in 2006 to $25.3 million in 2007 and again to $50.5 million in 2008. In the nine months ended September 30, 2009, our total revenues grew to $58.7 million from $31.9 million for the comparable period in 2008, which represents an 84% increase. Most of our revenues since inception have been derived from collaborative research and development arrangements, which accounted for 76%, 52% and 66% of our revenues in 2006, 2007 and 2008, respectively, and 77% of our revenues in the nine months ended September 30, 2009. Related party collaborative research and development received from Shell accounted for 33% and 60% of our revenues in 2007 and 2008, respectively, and 75% of our revenues in the nine months ended September 30, 2009. Our product sales have grown over the last three years, from $2.5 million in 2006 to $16.9 million in 2008. Notwithstanding our revenue growth, we have continued to experience significant losses as we have invested heavily in research and development and administrative infrastructure in connection with growth in our business. As of September 30, 2009, we had an accumulated deficit of $154.4 million. We incurred net losses of $18.7 million, $39.0 million, $45.1 million and $15.1 million in 2006, 2007, 2008 and the nine months ended September 30, 2009, respectively. In light of the growth in market acceptance of our products and services to date, we currently intend to increase our investment in research and development, such that we do not expect to achieve profitability prior to at least 2011.
We targeted the pharmaceutical industry as the first market for our products and services. In this market, we have historically entered into collaborations, which have involved complex service and intellectual property agreements under which we research and develop optimized biocatalysts for innovator pharmaceutical companies in connection with their drug development efforts. In these collaborations, we typically receive revenues in the form of one or more of the following: up-front payments, milestone
47
Table of Contents
payments, payments based upon the number of full-time employee equivalents, or FTEs, engaged in related research and development activities and licensing fees and royalties.
Our pharmaceutical product offerings include biocatalysts, pharmaceutical intermediates, active pharmaceutical ingredients, or APIs, and Codex Biocatalyst Panels. Our pharmaceutical customers incorporate our biocatalysts into the manufacturing processes used to produce their drugs. Our intermediates are complex chemical substances that have been manufactured by, or on behalf of, us using our biocatalysts. Drug manufacturers use intermediates to produce the APIs used in their drugs. We believe that major pharmaceutical manufacturers are increasingly willing to outsource portions of their own internal manufacturing and to purchase intermediates that are difficult or expensive to manufacture. Our Codex Biocatalyst Panels are plates embedded with genetically diverse variants of our proprietary biocatalysts, which allow our customers to screen our biocatalysts for desired activity that is applicable to a particular pharmaceutical manufacturing process. We view our Codex Biocatalyst Panels, which we began selling in 2007, as a way to build early and broad awareness of the power and utility of our technology platform. We plan to increase our efforts to expand use of our Codex Biocatalyst Panels among our current and potential customers.
Our pharmaceutical service offerings include screening and optimization services. We use our screening services to test our customers pharmaceutical materials against our existing libraries of biocatalysts to determine whether our existing biocatalysts produce any desired activities. We then use our optimization services to improve the performance of these biocatalysts to meet customer requirements. We also use our optimization services to improve biocatalysts identified by our customers through their use of our Codex Biocatalyst Panels. The use of our panels, as well as these services, has led to sales of biocatalysts to our pharmaceutical customers.
We provide our biocatalysts, Codex Biocatalyst Panels, screening and optimization services and intermediates to our innovator customers and provide intermediates to our generics customers. We have also launched several new intermediates and APIs for the generic equivalents of branded pharmaceutical products, including Singulair and Cymbalta, in markets where these products are not subject to patent protection, and intend to sell these same intermediates and APIs for use in other markets when the patent protection for each product expires. We sell our products primarily to pharmaceutical manufacturers through our small direct sales and business development force in the United States and Europe.
In the biofuels market, we entered into a research agreement with Shell in 2006. The goal of this collaboration was to develop biocatalysts to break down renewable sources of non-food plant materials, known as cellulosic biomass, and convert them to fuels. In connection with this collaboration, we received up-front payments, research and development service payments and a milestone payment.
Based on the success of this initial collaboration, in 2007, we entered into a new, expanded multi-year research and development collaboration with Shell to develop biocatalysts to convert cellulosic biomass into fermentable sugars that are used in the production of fuels and related products and to convert these sugars into fuels and related products. We received an up-front fee and are currently receiving FTE payments under this collaboration. This up-front fee is refundable under certain conditions, such as a change in control in which we are acquired by a competitor of Shell. This refundability lapses ratably over a five-year period beginning on November 1, 2007, on a straight-line basis. In 2009, we agreed to devote to the research collaboration FTEs by March 2009, which are required to be funded by Shell at an annual base rate per FTE of $ for FTEs located in the United States, and $ for FTEs located in Hungary. These annual base rates per FTE are subject to annual increases of each subsequent year of the collaboration. Until November 1, 2010, Shell has the right to reduce the number of funded FTEs under the collaborative research agreement by up to FTEs following 60 days advance written notice. After November 1, 2010, Shell has the right to further reduce the number of funded FTEs, with any one reduction not to exceed funded FTEs, following advance written notice. The required notice period ranges from 30 to 270 days, so the earliest an FTE reduction could take place would be December 2, 2010. Following any
48
Table of Contents
such reduction, Shell is subject to a standstill period of between 90 and 360 days during which period Shell cannot provide notice of any further FTE reductions. The notice and standstill periods are dependent on the number of funded FTEs reduced, with the length of notice and standstill periods increasing commensurate with the number of FTEs reduced.
We are also eligible for milestone payments of up to $ over the remaining term of the agreement, contingent upon the achievement of certain technical goals beginning in 2009, and a milestone payment of $ upon achievement of certain commercial goals. For the nine months ended September 30, 2009, we have met or exceeded each of our technical goals under the collaborative research agreement by the applicable deadlines and have earned a milestone payment of $0.5 million. In the fourth quarter of 2009, we became entitled to receive an additional $ million in milestone payments linked to achievement of technical goals. Shell will also be required to pay us a royalty per gallon with respect to certain products manufactured using our technology platform, including liquid fuels, fuel additives and lubricants, if Shell or any of its licensees manufactures such products. With respect to cellulosic biomass converted into sugars, Shell agreed to pay us a royalty per gallon of fuel product made from those sugars. With respect to sugars converted into fuel, Shell agreed to pay us a separate royalty per gallon of fuel product. We may be entitled to receive one or both of these royalties depending on whether Shell uses our technology to commercialize one or both of these steps.
Under our research and development collaboration with Shell, we retain ownership of all intellectual property we develop, other than patent rights related to certain fuel innovations, and Shell will have an exclusive license to such intellectual property we develop. We have agreed to work exclusively with Shell until November 2012 to convert cellulosic biomass into fermentable sugars that are used in the production of fuels and related products and to convert these sugars into fuels and related products. However, Shell is not required to work exclusively with us, and could develop or pursue alternative technologies that it decides to use for commercialization purposes instead of any technology developed under our collaborative research agreement. Even if Shell decides to commercialize products based on our technologies, they have no obligation to purchase their biocatalyst supply from us. If Shell chooses to commercialize any biofuels products developed through our collaboration, we believe that the combination of our technology platform with Shells proven project development capabilities and resources could enable a biofuels solution that extends from the conversion of cellulosic biomass into biofuels to delivery and distribution of refined biofuels to consumers at the pump.
One element of our collaboration with Shell relates to the development of cellulosic ethanol. In connection with our collaboration with Shell, we entered into a multi-party collaborative research and license agreement with Iogen Energy Corporation, or Iogen, and Shell in July 2009, which is focused on the conversion of cellulosic biomass to ethanol for commercial scale production. Iogen has agreed to pay us a royalty per gallon with respect to certain fuel products, which include liquid fuels, fuel additives and lubricants, that are covered by inventions jointly made by us and Iogen, but that are solely owned by Iogen. We will be entitled to collect royalties from Shell or Iogen for any use of our biofuels technology by Shell or Iogen. Shell can choose to commercialize cellulosic ethanol manufactured using our technology independently, or in collaboration with Iogen.
Under the terms of our license agreement with Maxygen, we are obligated to pay Maxygen a significant portion of certain types of consideration we receive in connection with our biofuels research and development, including our collaboration with Shell. The actual fees payable to Maxygen will depend on the amount, timing and type of consideration we receive, including payments from the sale of our equity securities to Shell and payments in connection with the sale of fuel products made with a biocatalyst developed using the licensed technology and/or research and development activities.
If we directly commercialize an energy product that is made using any biocatalyst developed from the technology licensed from Maxygen, we will owe Maxygen a 2% royalty on our net sales of the energy product and on amounts received from any sublicensee or third party for the use of the energy product, to
49
Table of Contents
the extent that we utilize such energy product to provide services to such sublicensee or third party. If we sublicense our rights under the license agreement to a third party for the development and commercialization of an energy product, we will owe Maxygen 20% of all consideration we receive from any sublicensee. Specifically, we will owe Maxygen fees in connection with consideration we receive in the form of (1) up-front option and/or license fees, (2) FTE funding for biofuels research, (3) milestone payments, (4) payments from the sale of our equity securities and (5) payments in connection with the commercialization of energy products made with a biocatalyst developed using the licensed technology.
In the case of consideration received from the sale of our equity securities to Shell, we are obligated to pay Maxygen 20% of any excess paid above $3.97 per share, the price per share of our Series D preferred stock. With regard to FTE funding, we are only obligated to pay Maxygen to the extent the consideration received exceeds a specified amount, which was initially $ per year starting in November 2006, but is adjusted annually by . We are also obligated to reimburse up to 20% of the costs incurred by Maxygen related to the prosecution and maintenance of the patents licensed from Maxygen relating to our core technology. Further, in the event that any subsidiary or affiliate of ours develops and/or sells any energy applications using the Maxygen technology, we are obligated to transfer to Maxygen a percentage of the value of the subsidiary or affiliate that is attributable to the Maxygen technology and give Maxygen an option to acquire a percentage of the other consideration that we invest in such affiliate or subsidiary.
In connection with all consideration received from Shell relating to our biofuels research and development collaboration, we were obligated to pay Maxygen $7.8 million and $1.0 million for 2007 and 2008, respectively, of which $ and $ , respectively, were payments owed to Maxygen in connection with Shells FTE funding. For the nine months ended September 30, 2008 and 2009, we were obligated to pay Maxygen $0.6 million and $4.2 million, respectively, of which $ and $ , respectively, were payments owed to Maxygen in connection with Shells FTE funding. The payments relating to FTE funding were less than 5% of the total FTE payments we received from Shell in those periods.
Our strategy for collaborative arrangements is to retain substantial participation in the future economic value of our technology while receiving current cash payments to offset research and development costs and working capital needs. These agreements are complex and have multiple elements that cover a variety of present and future activities. In addition, certain elements of these agreements are intrinsically difficult to separate and treat as separate units for accounting purposes, especially exclusivity payments. Consequently, we expect to recognize these exclusivity payments over the term of the exclusivity period.
We have limited internal manufacturing capacity at our headquarters in Redwood City, California. We expect to rely on third-party manufacturers for commercial production of our biocatalysts for the foreseeable future. Our in-house manufacturing is dedicated to producing both our Codex Biocatalyst Panels and biocatalysts for use by our customers in pilot scale production. We also supply initial commercial quantities of biocatalysts for use by our collaborators to produce pharmaceutical intermediates and manufacture biocatalysts that we sell.
We rely on two primary contract manufacturers, CPC Biotech srl, or CPC, and Lactosan GmbH & Co. KG, or Lactosan, to manufacture substantially all of the commercial biocatalysts used in our pharmaceutical business. We have qualified other contract manufacturers, but we do not currently use them for any of our supply commitments. In addition, we contract with other suppliers in Austria, Germany, Italy and India. Since 2006, Arch Pharmalabs Limited, or Arch, of Mumbai, India has manufactured all of our commercialized drug-related products for sale to generic API manufacturers. We are party to a number of agreements with Arch that govern the commercialization of various current and future products for supply into the generic and innovator marketplaces.
We continue to evaluate whether to develop internal capabilities to manufacture biocatalysts at commercial scale. To increase our biocatalyst manufacturing capacity, we may invest in our own manufacturing capabilities through the construction of additional manufacturing facilities. The factors we will consider in deciding whether to expand our internal manufacturing capabilities include the costs and
50
Table of Contents
impact on our cash flow associated with developing and maintaining such capabilities, the time required to develop such capabilities, potential locations for manufacturing sites, including proximity to existing customers, taxes associated with manufacturing activities and local incentives.
Our revenue stream is diversified across various industries, which should mitigate our exposure to cyclical downturns or fluctuations in any one market. Revenues during 2008 and the nine months ended September 30, 2009 were derived from the pharmaceuticals and biofuels markets, and consisted of collaborative research and development revenues, product sales and government grants, which are separately identified in our consolidated statements of operations. Based on our existing arrangements, we believe that revenues from both our pharmaceutical and biofuels customers should be predictable over the near term. The revenues that we expect to recognize from our collaborative research agreement with Shell should provide a high degree of visibility into our aggregate revenues for the foreseeable future.
We actively seek contract manufacturers who are willing to invest in capital equipment to manufacture our products at commercial scale. As a result, we are heavily dependent on the availability of manufacturing capacity at, and the reliability of, our contract manufacturers. We also pursue collaborations with industry leaders that allow us to leverage our collaborators engineering, manufacturing and commercial expertise, their distribution infrastructure and their ability to fund commercial scale production facilities. We believe that, if our collaborators choose to utilize our technology to commercialize new products, we expect our collaborators will finance, build and operate the larger, more expensive facilities for the intermediate or end products in our markets, which will allow us to expand into new markets without having to finance or operate large industrial facilities.
Revenues and Operating Expenses
Revenues
Our revenues are comprised of collaborative research and development revenues, product revenues and government grants.
| | Collaborative research and development revenues include license, technology access and exclusivity fees, FTE payments, milestones, royalties, and optimization and screening fees. We report our collaborative research and development revenues under two categories consisting of revenues (i) from related parties and (ii) from all other collaborators. Related party collaborative research and development revenues consist of revenues from Shell. |
| | Product revenues consist of sales of biocatalysts, intermediates and Codex Biocatalyst Panels. |
| | Government grants consist of payments from government entities. The terms of these grants generally provide us with cost reimbursement for certain types of expenditures in return for research and development activities over a contractually defined period. Historically, we have received government grants from Germany and the United States and expect to receive additional grants from other governments in the future. |
Cost of Product Revenues
Cost of product revenues includes both internal and third-party fixed and variable costs including amortization of purchased technology, materials and supplies, labor, facilities and other overhead costs associated with our product revenues.
Research and Development Expenses
Research and development expenses consist of costs incurred for internal projects as well as partner-funded collaborative research and development activities. These costs include license and royalty fees payable to Maxygen for consideration that we receive in connection with our biofuels collaboration, our direct and research-related overhead expenses, which include salaries and other personnel-related expenses,
51
Table of Contents
facility costs, supplies, depreciation of facilities, and laboratory equipment, as well as research consultants and the cost of funding research at universities and other research institutions, and are expensed as incurred. License and royalty fees payable to Maxygen may fluctuate depending on the timing and type of consideration received from Shell in connection with our biofuels research and development collaboration. Costs to acquire technologies that are utilized in research and development and that have no alternative future use are expensed when incurred. Our research and development efforts devoted to our internal product and process development projects increased significantly in 2007, 2008 and the nine months ended September 30, 2009, from 31 projects in 2006 to 46, 47 and 62 projects, respectively. Our internal research and development projects are typically completed in 12 to 24 months, and generally the costs associated with any single internal project during these periods were not material.
To approximate research and development expenses by funded category, we estimate, based on FTE efforts, the percentage of research and development efforts, as measured in hours incurred. The number of hours expended in each category is divided by the total number of hours expended on all categories of research and development with the resulting fractions then multiplied by the total cost of research and development effort, with the products then added to project-specific external costs. In the case where a collaborator is sharing the research and development costs, the expenses for that project are allocated proportionately between the collaborative projects funded by third parties and internal projects. We believe that presenting our research and development expenses in these categories will provide our investors with meaningful information on how our resources are being used.
The following table presents our approximate research and development expenses by funding category (in thousands):
| Years Ended December 31, | Nine Months Ended September 30, |
||||||||||||||
| 2006 | 2007 | 2008 | 2008 | 2009 | |||||||||||
| Collaborative research and development(1) |
$ | 4,150 | $ | 10,920 | $ | 17,368 | $ | 10,894 | $ | 28,554 | |||||
| Grants |
25 | 384 | 155 | 113 | | ||||||||||
| Internal projects |
13,082 | 24,340 | 28,031 | 22,243 | 10,932 | ||||||||||
| Total research and development expenses |
$ | 17,257 | $ | 35,644 | $ | 45,554 | $ | 33,250 | $ | 39,486 | |||||
| (1) | Research and development expenses related to collaborative projects funded by related and other third parties are less than the reported revenues due to the recognition of revenues from the amortization of non-refundable up-front payments. |
Selling, General and Administrative Expenses
Selling, general and administrative expenses consist of compensation expenses (including stock-based compensation), hiring and training costs, consulting and service provider expenses (including patent counsel related costs), marketing costs, occupancy-related costs, depreciation and amortization expenses and travel and relocation expenses.
Critical Accounting Policies and Estimates
The consolidated financial statements have been prepared in conformity with generally accepted accounting principles in the United States and include our accounts and the accounts of our wholly-owned subsidiaries. The preparation of our consolidated financial statements requires our management to make estimates, assumptions, and judgments that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the applicable periods. Management bases its estimates, assumptions and judgments on historical experience and on various other factors that are believed to be
52
Table of Contents
reasonable under the circumstances. Different assumptions and judgments would change the estimates used in the preparation of our consolidated financial statements, which, in turn, could change the results from those reported. Our management evaluates its estimates, assumptions and judgments on an ongoing basis.
The critical accounting policies requiring estimates, assumptions, and judgments that we believe have the most significant impact on our consolidated financial statements are described below.
Revenue Recognition
When evaluating multiple element arrangements, we consider whether the components of each arrangement represent separate units of accounting. Application of the standard requires subjective determinations and requires management to make judgments about the fair values of each individual element and whether it is separable from other aspects of the contractual relationship. Revenue arrangements with multiple components are divided into separate units of accounting if certain criteria are met, including whether the delivered component has stand-alone value to the customer, and whether there is objective and reliable evidence of the fair value of the undelivered items. Consideration received is allocated among the separate units of accounting based on their respective fair values. Applicable revenue recognition criteria are then applied to each of the units.
Revenues are recognized when the four basic revenue recognition criteria are met: (1) persuasive evidence of an arrangement exists; (2) products have been delivered, transfer of technology has been completed or services have been rendered; (3) the fee is fixed or determinable; and (4) collectibility is reasonably assured.
Our primary sources of revenues consist of collaborative research and development agreements, product revenues and government grants. Collaborative research and development agreements typically provide us with multiple revenue streams, including up-front fees for licensing, exclusivity and technology access, fees for FTE services and the potential to earn milestone payments upon achievement of contractual criteria and royalty fees based on future product sales or cost savings by our customers.
For each source of collaborative research and development revenues, product revenues and grant revenues, we apply the above revenue recognition criteria in the following manner:
| | Up-front fees received in connection with collaborative research and development agreements, including license fees, technology access fees and exclusivity fees, are deferred upon receipt, are not considered a separate unit of accounting and are recognized as revenues over the relevant performance periods under the agreements, as discussed below. |
| | Revenues related to FTE services are recognized as research services are performed over the related performance periods for each contract. We are required to perform research and development activities as specified in each respective agreement. The payments received are not refundable and are based on a contractual reimbursement rate per FTE working on the project. When up-front payments are combined with FTE services in a single unit of accounting, we recognize the up-front payments using the proportionate performance method of revenue recognition based upon the actual amount of research and development labor hours incurred relative to the amount of the total expected labor hours to be incurred by us, up to the amount of cash received. In cases where the planned levels of research services fluctuate substantially over the research term, we are required to make estimates of the total hours required to perform our obligations. Research and development expenses related to FTE services under the collaborative research and development agreements approximate the research funding over the term of the respective agreements. |
| | Revenues related to milestones that are determined to be substantive and at risk at the inception of the arrangement are recognized upon achievement of the milestone event and when collectability is reasonably assured. Milestone payments are triggered either by the results of our research efforts or by events external to us, such as our collaboration partner achieving a revenue target. Fees |
53
Table of Contents
| associated with milestones for which performance was not at risk at the inception of the arrangement or that are determined not to be substantive are accounted for in the same manner as the up-front fees, provided collectability is reasonably assured. |
| | We recognize revenues from royalties based on licensees sales of products using our technologies. Royalties are recognized as earned in accordance with the contract terms when royalties from licensees can be reasonably estimated and collectability is reasonably assured. |
| | Product revenues are recognized once passage of title and risk of loss has occurred and contractually specified acceptance criteria have been met, provided all other revenue recognition criteria have also been met. Product revenues consist of sales of biocatalysts, intermediates and APIs, and Codex Biocatalyst Panels. Cost of product revenues includes both internal and third party fixed and variable costs including amortization of purchased technology, materials and supplies, labor, facilities and other overhead costs associated with our product revenues. |
| | We license mutually agreed upon third party technology for use in our research and development collaboration with Shell. We record the license payments to research and development expense and offset related reimbursements received from Shell. Payments made by Shell to us are direct reimbursements of our costs. We account for these direct reimbursable costs as a net amount, whereby no expenses or revenues are recorded for the costs reimbursed by Shell. For any payments not reimbursed by Shell, we will recognize these as expenses in the statement of operations. We elected to present the reimbursement from Shell as a component of our research and development expense since presenting the receipt of payment from Shell as revenues does not reflect the substance of the arrangement. |
| | We receive payments from government entities in the form of government grants. Government grants are agreements that generally provide us with cost reimbursement for certain types of expenditures in return for research and development activities over a contractually defined period. Revenues from government grants are recognized in the period during which the related costs are incurred, provided that the conditions under which the government grants were provided have been met and we have only perfunctory obligations outstanding. |
| | Shipping and handling costs charged to customers are recorded as revenues. Shipping costs are included in our cost of product revenues. Such charges were not significant in any of the periods presented. |
Stock-Based Compensation
Prior to January 1, 2006, we accounted for stock-based employee compensation arrangements using the intrinsic value method required at the time. Under the intrinsic value method, compensation expense for employees is based on the intrinsic value of the option, determined as the excess, if any, of the fair value of the common stock over the exercise price of the option on the date of grant. Historically, our stock options have been granted with exercise prices at or above the estimated fair value of our common stock on the date of grant.
Effective January 1, 2006, we began recognizing compensation expense related to share-based transactions, including the awarding of employee stock options, based on the estimated fair value of the awards granted. We adopted this fair value method using the prospective transition method, as options granted prior to January 1, 2006 were measured using the minimum value method for the pro forma disclosures previously required. In accordance with the prospective transition method, we continued to account for non-vested employee share-based awards outstanding at the date of adoption using the intrinsic value method. All awards granted, modified or settled after January 1, 2006 have been accounted for using the fair value method.
54
Table of Contents
We account for stock options issued to non-employees based on their estimated fair value determined using the Black-Scholes option-pricing model. The fair value of the options granted to non-employees is remeasured as they vest, and the resulting increase in value, if any, is recognized as expense during the period the related services are rendered.
The following table summarizes the options granted from January 2008 through the date of this prospectus with their exercise prices, the fair value of the underlying common stock, and the intrinsic value per share, if any:
| Date of Issuance |
Number of Shares Subject to Options Granted |
Exercise Price per Share |
Fair Value of Common Stock per Share |
Intrinsic Value |
||||||||
| January 29, 2008 |
1,095,550 | $ | 7.00 | $ | 6.25 | $ | (0.75 | ) | ||||
| May 22, 2008 |
250,000 | 7.90 | 7.90 | | ||||||||
| September 25, 2008(1) |
10,000 | 4.57 | 7.19 | 2.62 | ||||||||
| September 25, 2008 |
750,012 | 7.19 | 7.19 | | ||||||||
| June 2, 2009 |
1,683,000 | 4.97 | 4.97 | | ||||||||
| August 5, 2009 |
376,495 | 4.93 | 4.93 | | ||||||||
| November 9, 2009 |
891,750 | 6.06 | 6.06 | | ||||||||
| 5,056,807 | ||||||||||||
| (1) | The exercise price of this stock option was the then-current fair value of our common stock when the employee joined our company, but such stock option was not issued until September 25, 2008, when the fair value of our common stock had increased to $7.19 per share. The stock option was subsequently cancelled, unexercised, shortly after grant when the employee left our company. |
Significant Factors, Assumptions and Methodologies Used in Determining Fair Value
We have estimated the fair value of our stock option grants on or after January 1, 2006 using the Black-Scholes option-pricing model. We calculate the estimated volatility rate based on selected companies in similar markets, due to a lack of historical information regarding the volatility of our stock price. We will continue to analyze the historical stock price volatility assumption as more historical data for our common stock becomes available. Due to our limited history of grant activity, we calculate the expected life of options granted to employees using the simplified method permitted by the SEC as the average of the total contractual term of the option and its vesting period. The risk-free rate assumption was based on U.S. Treasury instruments whose terms were consistent with the terms of our stock options. The expected dividend assumption was based on our history and expectation of dividend payouts. The fair value of the stock options granted was based on the following assumptions:
| Years ended December 31, | Nine months ended September 30, | |||||||||||
| 2007 | 2008 | 2008 | 2009 | |||||||||
| Weighted-average expected term (years) |
6.0 | 6.1 | 6.1 | 6.1 | ||||||||
| Weighted-average expected volatility |
48 | % | 57 | % | 57 | % | 75 | % | ||||
| Weighted-average risk-free interest rates |
4.3 | % | 3.2 | % | 3.2 | % | 2.5 | % | ||||
| Expected dividend yield |
0.0 | % | 0.0 | % | 0.0 | % | 0.0 | % | ||||
As a result of our Black-Scholes fair value calculations and the allocation of value to the vesting periods using the straight-line vesting attribution method, we recognized a total of $64,000 in stock-based
55
Table of Contents
compensation expense during 2006, of which $32,000 was attributable to employee stock options and $32,000 was attributable to non-employee stock options. Of these amounts, $61,000 was recorded as a selling, general and administrative expense while $3,000 was recorded as a research and development expense. We recognized a total of $1.3 million in stock-based compensation expense during 2007, of which $1.0 million was attributable to employee stock options and $0.2 million was attributable to non-employee stock options. Of these amounts, $0.8 million was recorded as a selling, general and administrative expense while $0.5 million was recorded as a research and development expense. We recognized a total of $3.5 million in stock-based compensation expense during 2008, of which $3.2 million was attributable to employee stock options and $0.3 million was attributable to non-employee stock options. Of these amounts, $2.0 million was recorded as general and administrative expense while $1.5 million was recorded as a research and development expense. In the nine months ended September 30, 2008 and 2009, we recognized a total of $2.4 million and $3.2 million in stock-based compensation expense, respectively, of which $2.1 million and $3.1 million, respectively, was attributable to employee stock options and $0.3 million and $0.1 million, respectively, was attributable to non-employee stock options. Of this total amount for the nine months ended September 30, 2008 and 2009, $1.4 million and $1.6 million, respectively, was recorded as a selling, general and administrative expense, while $1.0 million and $1.6 million, respectively, was recorded as a research and development expense.
Common Stock Valuations
The fair values of the common stock underlying our stock options were estimated contemporaneously by our board of directors with input from management based upon several factors, including progress and milestones attained in our business, projected sales and earnings for multiple future periods, and the probabilities of various financing and liquidation events, including winding up and dissolution. In determining the fair market value of our common stock as of the date of each option grant, our board of directors made a reasonable estimate of the then current value of our common stock. In the absence of a public trading market for our common stock, our board of directors was required to estimate the fair value of our common stock. Our board of directors considered numerous objective and subjective factors in determining the fair value of our common stock at each option grant date, including but not limited to the following factors: (i) prices of preferred stock issued by us primarily to outside investors in arms-length transactions, and the rights, preferences and privileges of the preferred stock relative to the common stock; (ii) our performance and the status of research and product development efforts; (iii) our stage of development and business strategy; and (iv) the likelihood of achieving a liquidity event for the shares of common stock underlying these stock options, such as an initial public offering or sale of our company, given then-prevailing market conditions.
All stock options were granted with exercise prices at or above the then-current fair market value of our common stock as determined by our board of directors, other than an option for 10,000 shares that was cancelled, unexercised, shortly after grant. We believe that the determinations of the value of our common stock were fair and reasonable at the time they were made. The board of directors utilized methodologies, approaches and assumptions consistent with the American Institute of Certified Public Accountants Practice Guide, Valuation of Privately-Held-Company Equity Securities Issued as Compensation, or the AICPA Practice Guide.
For our contemporaneous and retrospective valuations performed between December 2006 and September 2009 the board of directors used the probability-weighted expected return method, or the PWERM, which is consistent with the allocation methods outlined in the AICPA Practice Guide. The PWERM analyzes the returns afforded to common equity holders under multiple future scenarios. Under the PWERM, share value is based upon the probability-weighted present value of expected future net cash flows (distributions to shareholders), considering each of the possible future events and giving consideration for the rights and preferences of each share class. The PWERM requires a five step process: (i) for each possible future event, standard valuation methodologies, such as the application of revenues and earnings multiples from a relevant peer group, are used
56
Table of Contents
to estimate a range of future distribution values over a range of event dates; (ii) for each combination of value and date, the value is allocated between the share classes; (iii) the expected return for each class is then discounted back to the present; (iv) the probability for each possible event is estimated; and (v) the probability-weighted return, expressed in terms of a per-share value, is determined for each class. Although this method is complex to implement, the board of directors believes that this methods forward-looking analysis of potential future outcomes makes it the most suitable for this analysis.
The PWERM-derived fair value calculated at each valuation date was then allocated to the shares of redeemable and/or convertible preferred stock, warrants to purchase shares of preferred stock, and common stock, using a contingent claim methodology. This methodology treats the various components of our capital structure as a series of call options on the proceeds expected from the sale of the company or the liquidation of our assets at some future date. The anticipated timing of a liquidity event utilized in these valuations was based on the then-current plans and estimates of our board of directors and management regarding the likely success of an initial public offering. Estimates of the volatility of our stock were based on the limited information available on the volatility of the capital stock of comparable publicly-traded companies.
We granted stock options with exercise prices between $4.93 and $6.06 per share during 2009. We granted stock options with exercise prices between $4.57 and $7.90 per share during 2008. No single event caused the valuation of our common stock to increase or decrease from January 2008 to September 2009; rather, it has been a combination of the following factors that led to the changes in the fair value of the underlying common stock:
January 2008: In January 2008, we appointed a new President for Codexis Pharmaceuticals, opened a new European facility in Hungary and introduced a new product. Also, our board of directors selected investment banks to act as managing underwriters for a potential initial public offering of our stock. As a result of these events, on January 29, 2008, the fair value of our common stock was estimated to be $6.25 per share.
February 2008 to May 2008: In April 2008, we filed a registration statement on Form S-1 with the SEC for a potential initial public offering of our common stock. As a result, on May 22, 2008, the estimated fair value of our common stock increased to $7.90 per share.
May 2008 to June 2008: In June, we entered into two new collaborative research agreements to provide our Codex Biocatalyst Panels and screening services. As a result, on June 30, 2008, the estimated fair value of our common stock increased to $8.10 per share.
July 2008 to September 2008: In September 2008, we determined market conditions had deteriorated and volatility had increased and we filed to withdraw our registration statement on Form S-1 with the SEC. We deemed the probability of an initial public offering to have significantly decreased in the near term. We also announced an expansion of our agreement with Arch. However, due primarily to the conditions in the equity markets which had led to the withdrawal of our earlier registration statement, as of September 25, 2008, the estimated fair value of our common stock decreased to $7.19 per share.
October 2008 to December 2008: In November, we announced a technology license agreement with Dyadic International. We also began discussions with Shell and other potential investors regarding a Series F preferred stock financing. Due to prevailing market conditions, we determined it was highly unlikely that an initial public offering would be consummated in 2009. As a result of such conditions, on December 31, 2008, the estimated fair value of our common stock decreased to $5.42 per share.
January 2009 to March 2009: In March 2009, we completed the first closing of our Series F preferred stock financing, led by Shell, raising $30.0 million. We also expanded our amended and restated collaborative research agreement with Shell. Despite these events, because of the conditions in the equity markets, as of March 31, 2009, the estimated fair value of our common stock decreased to $4.96 per share.
57
Table of Contents
April 2009 to July 2009: In May 2009, we appointed a Senior Vice President of Research and Development and a Chief Science Officer. We announced an agreement with F. Hoffman-La Roche Ltd., or Roche, under which Roche will purchase our Codex Biocatalyst Panels. We raised $15.0 million through additional closings of sales of our Series F preferred stock. Although revenues were up 105% for the first seven months of 2009 compared to 2008, we were still recording losses during this period. As a result of the dilutive effect from having additional potential common shares as compared to the prior valuation, the estimated fair value of our common stock decreased to $4.93 per share.
August 2009 to September 2009: In August 2009, we underwent certain restructuring activities which included closing our German facility and relocating operations into other facilities. By late August 2009, conditions in the equity markets had improved and continued to improve into September 2009. Based on these events, on September 29, 2009, the estimated fair value of our common stock increased to $6.06 per share.
Estimation of Fair Value of Warrants to Purchase Preferred Stock
Our outstanding warrants to purchase shares of our preferred stock are required to be classified as current liabilities and to be adjusted to their fair value at the end of each reporting period. Warrants issued in connection with debt arrangements resulted in an aggregate expense of $0.2 million and $1.3 million attributable to an increase in the fair value of the warrant liability recognized in interest expense and other, net in the consolidated statements of operations during 2006 and 2007, respectively. For the year ended December 31, 2008, a gain of $0.1 million was recognized in interest expense and other, net as a result of warrant liability measurement. During the nine months ended September 30, 2008 and 2009, losses of $0.6 million and $0.3 million, respectively, were recognized in interest expense and other, net due to the warrant liability remeasurement. Upon the closing of this initial public offering and the conversion of the underlying preferred stock to common stock, all outstanding warrants to purchase shares of preferred stock will automatically convert into warrants to purchase shares of our common stock. The then-current aggregate fair value of these warrants will be reclassified from liabilities to additional paid-in capital, a component of stockholders equity, and we will cease to record any related periodic fair value adjustments. Accordingly, we estimated the fair value of these warrants on an as-if converted basis at the respective balance sheet dates using the Black-Scholes option pricing model, the remaining contractual term of the warrant, risk-free interest rates and expected dividends on and expected volatility of the price of the underlying common stock. These estimates, especially the market value of the underlying common stock and the expected volatility, are highly judgmental and could differ materially in the future.
Impairment of Goodwill and Intangible Assets and Other Long-lived Assets
We assess impairment of long-lived assets, including goodwill, on at least an annual basis and test long-lived assets for recoverability when events or changes in circumstances indicate that their carrying amount may not be recoverable. Circumstances which could trigger a review include, but are not limited to: significant decreases in the market price of the asset; significant adverse changes in the business climate or legal factors; accumulation of costs significantly in excess of the amount originally expected for the acquisition or construction of the asset; current period cash flow or operating losses combined with a history of losses or a forecast of continuing losses associated with the use of the asset; or current expectation that the asset will more likely than not be sold or disposed of significantly before the end of its estimated useful life.
Recoverability is assessed based on the sum of the undiscounted cash flows expected to result from the use and the eventual disposal of the asset. An impairment loss is recognized in the consolidated statements of operations when the carrying amount is not recoverable and exceeds fair value, which is determined on a discounted cash flow basis.
58
Table of Contents
We make estimates and judgments about future undiscounted cash flows and fair value. Although our cash flow forecasts are based on assumptions that are consistent with our plans, there is significant exercise of judgment involved in determining the cash flows attributable to a long-lived asset over its estimated remaining useful life. Our estimates of anticipated future cash flows could be reduced significantly in the future. As a result, the carrying amount of our long-lived assets could be reduced through impairment charges in the future. Changes in estimated future cash flows could also result in a shortening of estimated useful life of long-lived assets including intangibles for depreciation and amortization purposes.
Income Tax Provision
We use the liability method of accounting for income taxes, whereby deferred tax assets or liability account balances are calculated at the balance sheet date using current tax laws and rates in effect for the year in which the differences are expected to affect taxable income. Valuation allowances are provided when necessary to reduce deferred tax assets to the amount that will more likely than not be realized.
We must make certain estimates and judgments in determining income tax expense for financial statement purposes. These estimates and judgments occur in the calculation of tax credits, benefits and deductions and in the calculation of certain tax assets and liabilities, which arise from differences in the timing of recognition of revenues and expenses for tax and financial statement purposes. Significant changes to these estimates may result in an increase or decrease to our tax provision in a subsequent period.
In assessing the realizability of deferred tax assets, we consider whether it is more likely than not that some portion or all of the deferred tax assets will be realized on a jurisdiction by jurisdiction basis. The ultimate realization of deferred tax assets is dependent upon the generation of taxable income in the future. We have recorded a deferred tax asset in jurisdictions where ultimate realization of deferred tax assets is more likely than not to occur.
We make estimates and judgments about our future taxable income that are based on assumptions that are consistent with our plans and estimates. Should the actual amounts differ from our estimates, the amount of our valuation allowance could be materially impacted. Any adjustment to the deferred tax asset valuation allowance would be recorded in the income statement for the periods in which the adjustment is determined to be required.
On January 1, 2007, we adopted the Financial Accounting Standards Board, or FASB, standard for accounting for uncertainty in income taxes. The revised standard, now codified under the Income Taxes Topic in the FASB Accounting Standards Codification clarifies the accounting for uncertainty in income taxes recognized in an enterprises financial statements. The first step is to evaluate the tax position for recognition by determining if the weight of available evidence indicates that it is more likely than not that the position will be sustained on audit, including resolution of related appeals or litigation processes, if any. The second step is to estimate and measure the tax benefit as the largest amount that is more than 50% likely of being realized upon ultimate settlement. It is inherently difficult and subjective to estimate such amounts, as this requires us to determine the probability of various possible outcomes. We consider many factors when evaluating and estimating our tax positions and tax benefits, which may require periodic adjustments and may not accurately anticipate actual outcomes.
59
Table of Contents
Results of Operations
Nine Months Ended September 30, 2008 and 2009
The following table shows the amounts and percentage relationships of the listed items from our unaudited consolidated statements of operations for the periods presented, showing period-over-period changes (in thousands, except for percentages).
| Nine Months Ended September 30, |
$ Change | % Change | |||||||||||||
| 2008 | 2009 | ||||||||||||||
| Revenues: |
|||||||||||||||
| Product |
$ | 10,777 | $ | 13,401 | $ | 2,624 | 24 | % | |||||||
| Related party collaborative research and development |
18,174 | 43,963 | 25,789 | 142 | |||||||||||
| Collaborative research and development |
2,678 | 1,295 | (1,383 | ) | (52 | ) | |||||||||
| Government grants |
251 | 11 | (240 | ) | (96 | ) | |||||||||
| Total revenues |
31,880 | 58,670 | 26,790 | 84 | |||||||||||
| Costs and operating expenses: |
|||||||||||||||
| Cost of product revenues |
7,922 | 11,886 | 3,964 | 50 | |||||||||||
| Research and development |
33,250 | 39,486 | 6,236 | 19 | |||||||||||
| Selling, general and administrative |
28,300 | 20,939 | (7,361 | ) | (26 | ) | |||||||||
| Total costs and operating expenses |
69,472 | 72,311 | 2,839 | 4 | |||||||||||
| Loss from operations |
(37,592 | ) | (13,641 | ) | 23,951 | (64 | ) | ||||||||
| Interest income |
1,435 | 141 | (1,294 | ) | (90 | ) | |||||||||
| Interest expense and other, net |
(2,517 | ) | (1,530 | ) | 987 | (39 | ) | ||||||||
| Loss before provision for income taxes |
(38,674 | ) | (15,030 | ) | 23,644 | (61 | ) | ||||||||
| Provision for income taxes |
126 | 79 | (47 | ) | (37 | ) | |||||||||
| Net loss |
$ | (38,800 | ) | $ | (15,109 | ) | $ | 23,691 | (61 | )% | |||||
Revenues. Revenues increased $26.8 million, or 84%, from $31.9 million in the nine months ended September 30, 2008 to $58.7 million in the nine months ended September 30, 2009, primarily due to increases in revenues from related party collaborative research and development projects and product sales.
Product revenues increased $2.6 million, or 24%, from $10.8 million in the nine months ended September 30, 2008 to $13.4 million in the nine months ended September 30, 2009. This increase was primarily due to an increase in product sales to a pharmaceutical customer during the nine months ended September 30, 2009.
Related party collaborative research and development revenues increased $25.8 million, or 142%, from $18.2 million in the nine months ended September 30, 2008 to $44.0 million in the nine months ended September 30, 2009. This increase was due to the increase in the number of FTEs engaged in our expanded research and development collaboration with Shell. The expansion of this collaboration resulted in an increase in the number of contractual FTEs used during the period from an average of in the nine months ended September 30, 2008 to an average of in the nine months ended September 30, 2009.
Collaborative research and development revenues decreased $1.4 million, or 52%, from $2.7 million in the nine months ended September 30, 2008 to $1.3 million in the nine months ended September 30, 2009. This decrease was primarily due to the reallocation of our research resources after the completion of certain collaborative research and development projects to related party collaborative research and development projects.
60
Table of Contents
Government grant revenues decreased $0.2 million, or 96%, from $0.3 million in the nine months ended September 30, 2008 to $11,000 in the nine months ended September 30, 2009.
Our top five customers accounted for 76% and 90% of our total revenues in the nine months ended September 30, 2008 and 2009, respectively. In the nine months ended September 30, 2008, Shell accounted for 57% of our total revenues. In the nine months ended September 30, 2009, Shell accounted for 75% of our total revenues.
Customers in the Americas accounted for 69% and 82% of our revenues, and customers outside the Americas accounted for 31% and 18% of our revenues, in the nine months ended September 30, 2008 and 2009, respectively. Revenues for the nine months ended September 30, 2008 and 2009 by geography were as follows (in thousands, except percentages):
| Nine Months Ended September 30, |
$ Change | % Change | ||||||||||
| 2008 | 2009 | |||||||||||
| Americas(1) |
$ | 22,017 | $ | 48,110 | $ | 26,093 | 119 | % | ||||
| Europe |
5,934 | 6,146 | 212 | 4 | ||||||||
| Asia |
3,929 | 4,414 | 485 | 12 | ||||||||
| International |
9,863 | 10,560 | 697 | 7 | ||||||||
| Total |
$ | 31,880 | $ | 58,670 | $ | 26,790 | 84 | % | ||||
| (1) | Primarily United States. |
Cost of Product Revenues. Cost of product revenues was $7.9 million for the nine months ended September 30, 2008, compared to $11.9 million in the nine months ended September 30, 2009, an increase of $4.0 million. The increase was primarily attributable to product sales. Cost of product revenues as a percentage of product revenues increased from 74% in the nine months ended September 30, 2008 to 89% in the nine months ended September 30, 2009, primarily due to write downs of $1.4 million of inventory items, as well as a change in sales mix towards lower margin product sales during the nine months ended September 30, 2009. Inventory write downs included excess and obsolete inventories and the impact of the rationalization of our product offerings in connection with the closure of our facility in Germany.
Research and Development. Research and development expenses were $33.3 million in the nine months ended September 30, 2008, compared to $39.5 million in the nine months ended September 30, 2009, an increase of $6.2 million or 19%. The increase was primarily due to increased royalty fees paid to Maxygen of $3.7 million, most of which was related to Shells increased equity investment in our company, and the remainder of which reflected the increase in FTEs. In addition, the increase was due to compensation (including stock-based compensation) and benefits of $2.5 million attributable to an increase in employee headcount in our research and development functions, and depreciation and amortization expense of $1.1 million due to expanded facilities and capital equipment. These costs were offset by reductions in supplies, consultants and other costs of $1.1 million. Research and development expenses included stock-based compensation expense of $1.0 million and $1.6 million during the nine months ended September 30, 2008 and 2009, respectively.
Selling, General and Administrative. Selling, general and administrative expenses were $28.3 million for the nine months ended September 30, 2008, compared to $20.9 million for the nine months ended September 30, 2009, a decrease of $7.4 million or 26%. The decrease was primarily due to a $3.6 million write off in 2008 of deferred initial public offering costs. We also incurred lower costs for consultants, contractors and outside advisory services of $1.7 million, and travel and recruiting-related expenses decreased by $0.9 million. Selling, general and administrative expenses included stock-based compensation expense of $1.3 million and $1.6 million during the nine months ended September 30, 2008 and 2009, respectively.
61
Table of Contents
Interest Income. Interest income was $1.4 million in the nine months ended September 30, 2008 compared to $0.1 million in the nine months ended September 30, 2009, a decrease of $1.3 million, or 90%. The decrease resulted from higher average cash, cash equivalents and marketable securities balances on hand and higher average interest rates during the first nine months of 2008 compared to the first nine months of 2009.
Interest Expense and Other, Net. Interest expense and other, net was $2.5 million in the nine months ended September 30, 2008, compared to $1.5 million in the nine months ended September 30, 2009, a decrease of $1.0 million or 39%. Interest expense and other, net in the nine months ended September 30, 2009 included the increase in the fair value of our redeemable convertible preferred stock warrants of $0.3 million, and a decrease in interest expense of $0.5 million due to the reduced debt obligation on the General Electric Capital Corporation / Oxford Finance Corporation loan, which we refer to as the GE Capital Loan, due to scheduled principal payments on these obligations. Interest expense and other, net in the nine months ended September 30, 2008 included the increase in the fair value of the redeemable convertible preferred stock warrants of $0.6 million, and higher interest expense due to higher debt obligations on the GE Capital Loan.
Provision for Income Taxes. The tax provision for the nine months ended September 30, 2008 and 2009 primarily consisted of foreign taxes withheld at source on royalties earned overseas and other taxes attributable to foreign operations.
Restructuring Charges. In 2009, our board of directors approved and committed to plans to reduce our cost structure, which included a relocation of our operations in Germany to facilities in the United States and in Singapore, a rationalization of our product offerings, closure of the facility in Germany and employee terminations in Germany and the United States. We expensed $0.2 million in employee severance and benefits and $0.5 million related to inventory write downs, for a total of $0.8 million. These costs were included in cost of product revenues in the consolidated statements of operations. As of September 30, 2009, $0.6 million related to these expenses has been paid or charged off and the remaining $0.2 million is recorded in other accrued liabilities on the consolidated balance sheet. We anticipate total costs of the plans to be approximately $1.4 million, with substantially all of the remaining $0.6 million (related to lease termination and employee severance and benefits) expected to be incurred by December 31, 2009.
62
Table of Contents
Years Ended December 31, 2007 and 2008
The following table shows the amounts and percentage relationships of the listed items from our consolidated statements of operations for the periods presented, showing period-over-period changes (in thousands, except percentages).
| 2007 | 2008 | $ Change | % Change | ||||||||||||
| Revenues: |
|||||||||||||||
| Product |
$ | 11,418 | $ | 16,860 | $ | 5,442 | 48 | % | |||||||
| Related party collaborative research and development |
8,481 | 30,239 | 21,758 | 257 | |||||||||||
| Collaborative research and development |
4,733 | 3,062 | (1,671 | ) | (35 | ) | |||||||||
| Government grants |
701 | 317 | (384 | ) | (55 | ) | |||||||||
| Total revenues |
25,333 | 50,478 | 25,145 | 99 | |||||||||||
| Costs and operating expenses: |
|||||||||||||||
| Cost of product revenues |
8,319 | 13,188 | 4,869 | 59 | |||||||||||
| Research and development |
35,644 | 45,554 | 9,910 | 28 | |||||||||||
| Selling, general and administrative |
19,713 | 35,709 | 15,996 | 81 | |||||||||||
| Total costs and operating expenses |
63,676 | 94,451 | 30,775 | 48 | |||||||||||
| Loss from operations |
(38,343 | ) | (43,973 | ) | (5,630 | ) | 15 | ||||||||
| Interest income |
1,491 | 1,538 | 47 | 3 | |||||||||||
| Interest expense and other, net |
(2,533 | ) | (2,365 | ) | 168 | (7 | ) | ||||||||
| Loss before provision (benefit) for income taxes |
(39,385 | ) | (44,800 | ) | (5,415 | ) | 14 | ||||||||
| Provision (benefit) for income taxes |
(408 | ) | 327 | 735 | NM | ||||||||||
| Net loss |
$ | (38,977 | ) | $ | (45,127 | ) | $ | (6,150 | ) | 16 | % | ||||
NM = not meaningful
Revenues. From 2007 to 2008, revenues increased $25.1 million, or 99%, from $25.3 million to $50.5 million due primarily to increases in revenues from related party collaborative research and development projects and product sales.
Product revenues increased $5.4 million, or 48%, from $11.4 million in 2007 to $16.9 million in 2008. This increase was primarily due to a $4.4 million increase in sales of intermediates which began in the first quarter of 2008, and a $1.1 million increase in biocatalyst sales.
Related party collaborative research and development revenues increased $21.8 million, or 257%, from $8.5 million in 2007 to $30.2 million in 2008. This increase was due to the expansion of the research and development collaboration with Shell that took place during 2008. The expansion of this collaboration resulted in an increase in the number of contractual FTEs used during the year from an average of in 2007 to an average of in 2008.
Collaborative research and development revenues decreased $1.7 million, or 35%, from $4.7 million in 2007 to $3.1 million in 2008. This decrease was primarily due to a $2.4 million decrease as a result of completion of collaboration projects with two pharmaceutical customers during 2007, partially offset by a $0.7 million increase as a result of optimization services delivered to one pharmaceutical customer and additional royalties received from another pharmaceutical customer.
Government grant revenues decreased $0.4 million, or 55%, from $0.7 million in 2007 to $0.3 million in 2008. This decrease was primarily due to the completion of a grant received from the National Institutes of Health at the end of 2007.
63
Table of Contents
Our top five customers accounted for 65% and 79% of total revenues for 2007 and 2008, respectively. In 2007, Shell accounted for 33% of our total revenues and Pfizer accounted for 13% of our total revenues. In 2008, Shell accounted for 60% of our total revenues and no other customer accounted for more than 10% of our total revenues.
Customers in the Americas accounted for 59% and 70% of revenues and customers outside the Americas accounted for 41% and 30% of revenues in 2007 and 2008, respectively. Revenues for 2007 and 2008 by geography were as follows (in thousands, except for percentages):
| 2007 | 2008 | $ Change | % Change | |||||||||
| Americas(1) |
$ | 15,010 | $ | 35,166 | $ | 20,156 | 134 | % | ||||
| Europe |
4,005 | 8,165 | 4,160 | 104 | ||||||||
| Asia |
6,318 | 7,147 | 829 | 13 | ||||||||
| International |
10,323 | 15,312 | 4,989 | 48 | ||||||||
| Total |
$ | 25,333 | $ | 50,478 | $ | 25,145 | 99 | % | ||||
| (1) | Primarily United States. |
Cost of Product Revenues. Cost of product revenues was $8.3 million for 2007 compared to $13.2 million in 2008, an increase of $4.9 million or 59%. The increase was primarily attributable to the 48% increase in product sales. In addition, cost of product revenues as a percentage of product revenues increased from approximately 73% in 2007 to 78% in 2008 due to a change in sales mix towards lower margin product sales in 2008.
Research and Development. Research and development expenses increased from $35.6 million for 2007 to $45.6 million for 2008, an increase of $9.9 million or 28%. The increase was primarily due to increased compensation (including stock-based compensation) and benefits of $10.5 million attributable to a 27% increase in employee headcount in our research and development functions, higher expenses incurred for lab supplies, outside services and consultants of $4.2 million, higher occupancy related costs of $1.3 million and depreciation and amortization expense of $1.4 million. These increases were partially offset by a $7.0 million decrease in fees payable to Maxygen in connection with the receipt of an up-front payment during 2007 related to our research and development collaboration with Shell. Research and development expenses included stock-based compensation expense of $0.5 million and $1.5 million during 2007 and 2008, respectively.
Selling, General and Administrative. Selling, general and administrative expenses increased from $19.7 million for 2007 to $35.7 million for 2008, an increase of $16.0 million or 81%. The increase was primarily due to increased compensation (including stock-based compensation) of $3.4 million attributable to a 45% increase in our employee headcount, primarily related to our accounting, legal, information technology and sales departments. In addition, we incurred higher costs during 2008 for consultants and outside advisory services, including $4.0 million as we prepared to become a public company and $2.4 million in patent protection costs. Also, in 2008, we expensed $3.6 million in initial public offering costs which had been deferred until the initial public offering was withdrawn in September 2008. Restructuring charges included in selling, general and administrative expenses in 2008 were $2.0 million. Expenses related to promotional marketing materials and travel increased $0.8 million. Selling, general and administrative expenses included stock-based compensation expense of $0.8 million and $2.0 million during 2007 and 2008, respectively.
Interest Income. Interest income was $1.5 million in both 2007 and 2008.
Interest Expense and Other, Net. Interest expense and other, net was $2.5 million in 2007 compared to $2.4 million in 2008, or a decrease of $0.2 million or 7%. Interest expense and other, net in 2007 included a $1.3 million expense related to the increase in the fair value of our Series D redeemable
64
Table of Contents
convertible preferred stock warrants. The increase in interest expense in 2008 was $1.2 million and was related to the outstanding principal on the GE Capital Loan that was drawn in September 2007.
Provision (Benefit) for Income Taxes. The tax provision for 2008 primarily consisted of foreign tax withheld at source on royalties earned overseas and other taxes attributable to foreign operations. The tax benefit for 2007 primarily consisted of benefit from reductions in deferred tax liabilities that had originated in a business acquisition, offset by foreign tax withheld at source on royalties earned overseas and other taxes attributable to foreign operations.
Restructuring Charges. In 2008, our board of directors approved and committed to plans to reduce our cost structure. The restructuring plan applied to employees and facilities worldwide. We expensed $1.1 million for facilities, $0.6 million for employees and $0.2 million in other costs associated with the closure of the Pasadena site for a total of $2.0 million in the year ended December 31, 2008. Restructuring expense was included in general and administrative expenses in the consolidated statements of operations. As of December 31, 2008, $0.4 million has been paid and the remaining expenses are recorded on the consolidated balance sheet in other accrued liabilities for $0.8 million and in other long-term liabilities for $0.7 million. During the nine months ended September 30, 2009, $1.3 million was paid, and $0.1 million was reversed as reduction of general and administrative expense due to a change in estimated costs of restructuring. The amounts included in other accrual liabilities on the consolidated balance sheet as of September 30, 2009 under this restructuring plan were $0.1 million.
Years Ended December 31, 2006 and 2007
The following table shows the amounts and percentage relationships of the listed items from our consolidated statements of operations for the periods presented, showing period-over-period changes (in thousands, except percentages).
| 2006 | 2007 | $ Change | % Change | ||||||||||||
| Revenues: |
|||||||||||||||
| Product |
$ | 2,544 | $ | 11,418 | $ | 8,874 | 349 | % | |||||||
| Related party collaborative research and development |
863 | 8,481 | 7,618 | 883 | |||||||||||
| Collaborative research and development |
8,403 | 4,733 | (3,670 | ) | (44 | ) | |||||||||
| Government grants |
317 | 701 | 384 | 121 | |||||||||||
| Total revenues |
12,127 | 25,333 | 13,206 | 109 | |||||||||||
| Costs and operating expenses: |
|||||||||||||||
| Cost of product revenues |
1,806 | 8,319 | 6,513 | 361 | |||||||||||
| Research and development |
17,257 | 35,644 | 18,387 | 107 | |||||||||||
| Selling, general and administrative |
11,880 | 19,713 | 7,833 | 66 | |||||||||||
| Total costs and operating expenses |
30,943 | 63,676 | 32,733 | 106 | |||||||||||
| Loss from operations |
(18,816 | ) | (38,343 | ) | (19,527 | ) | 104 | ||||||||
| Interest income |
742 | 1,491 | 749 | 101 | |||||||||||
| Interest expense and other, net |
(724 | ) | (2,533 | ) | (1,809 | ) | 250 | ||||||||
| Loss before income tax benefit |
(18,798 | ) | (39,385 | ) | (20,587 | ) | 110 | ||||||||
| Income tax benefit |
(127 | ) | (408 | ) | (281 | ) | 221 | ||||||||
| Net loss |
$ | (18,671 | ) | $ | (38,977 | ) | $ | (20,306 | ) | 109 | % | ||||
Revenues. From 2006 to 2007, revenues increased $13.2 million, or 109%, from $12.1 million to $25.3 million due primarily to increases in revenues from related party collaborative research and development projects and product sales.
65
Table of Contents
Product revenues increased $8.9 million, or 349%, from $2.5 million to $11.4 million in 2006 and 2007, respectively. This increase was primarily due to a $4.2 million increase in sales of our ATS-8 intermediate to manufacturers of generic atorvastatin, $1.7 million of additional sales from our new BioCatalytics subsidiary, and a $2.9 million increase in sales of our other biocatalyst and intermediate products.
Related party collaborative research and development revenues increased $7.6 million, or 883%, from $0.9 million to $8.5 million in 2006 and 2007, respectively. This increase was primarily due to the expanded research and development collaboration with Shell.
Collaborative research and development revenues decreased $3.7 million, or 44%, from $8.4 million to $4.7 million in 2006 and 2007, respectively. This decrease was primarily due to the reallocation of research and development resources to related party collaborative research and development projects.
Government grant revenues increased $0.4 million, or 121%, from $0.3 million to $0.7 million in 2006 and 2007, respectively. This increase was due to a $0.3 million grant received from the National Institutes of Health and an additional $0.1 million grant received from the German government.
Our top five customers accounted for 71% and 65% of total revenues for 2006 and 2007, respectively. In 2006, Pfizer accounted for 36% of our revenues and Schering-Plough accounted for 11% of our revenues. In 2007, Shell accounted for 33% of our revenues and Pfizer accounted for 13% of our revenues.
Customers in the Americas accounted for 65% and 59% of revenues, and customers outside the Americas accounted for 35% and 41% of revenues, in 2006 and 2007, respectively. Revenues for 2006 and 2007 by geography were as follows (in thousands, except for percentages):
| 2006 | 2007 | $ Change | % Change | |||||||||
| Americas(1) |
$ | 7,933 | $ | 15,010 | $ | 7,077 | 89 | % | ||||
| Europe |
2,491 | 4,005 | 1,514 | 61 | ||||||||
| Asia |
1,703 | 6,318 | 4,615 | 271 | ||||||||
| International |
4,194 | 10,323 | 6,129 | 146 | ||||||||
| Total |
$ | 12,127 | $ | 25,333 | $ | 13,206 | 109 | % | ||||
| (1) | Primarily United States. |
Cost of Product Revenues. Cost of product revenues was $1.8 million for 2006 compared to $8.3 million in 2007, an increase of $6.5 million, or 361%. The increase was primarily attributable to the increase in product sales of $8.9 million, an increase in amortization of intangible assets of $0.1 million and an inventory fair value adjustment related to our BioCatalytics acquisition of $0.2 million. Cost of product revenues as a percentage of product revenues increased from 71% in 2006 to 73% in 2007.
Research and Development. Research and development expenses were $17.3 million in 2006 compared to $35.6 million in 2007, an increase of $18.4 million, or 107%. The increase was primarily due to increased royalty costs of $7.3 million due to Maxygen in connection with amounts received from Shell relating to our biofuels research and development collaboration, and increased compensation (including stock-based compensation) benefits, hiring and training costs of $6.8 million attributable to an increase in employee headcount in our research and development functions. Also reflecting this increased research activity were higher expenses incurred for lab supplies, outside services and consultants of $2.2 million, plus higher occupancy related costs of $0.9 million and depreciation and amortization expense of $0.4 million. Travel and relocation expenses were also higher by $0.8 million as our research functions expanded in Europe and Asia. Professional and advisory fees also increased by $0.6 million. Included in the above amounts were $3.5 million of additional research and development expenses that we incurred after opening our new research facility in Singapore in October 2007. Research and development expenses included stock-based compensation expense of $3,000 and $0.5 million during 2006 and 2007, respectively.
66
Table of Contents
Selling, General and Administrative. Selling, general and administrative expenses were $11.9 million for 2006, compared to $19.7 million for 2007, an increase of $7.8 million, or 66%. The increase was primarily due to increased compensation (including stock-based compensation) of $2.8 million attributable to higher employee headcount. We incurred higher costs for consultants and outside advisory services of $1.5 million as we prepared to become a public company, including consulting costs associated with preparation for Sarbanes-Oxley compliance. We also incurred higher professional fees of $1.0 million in 2007 mainly for legal costs connected with negotiating our collaboration agreements and other contracts during the year. Expenses related to promotional marketing materials and travel increased $0.6 million. Selling, general and administrative expenses included stock-based compensation expense of $0.1 million and $0.8 million during 2006 and 2007, respectively.
Interest Income. Interest income was $0.7 million in 2006 compared to $1.5 million in 2007, an increase of $0.7 million, or 101%. The increase resulted from the higher average cash and investment balances on hand during 2007 compared to 2006. These higher cash and investment balances resulted from cash received in connection with the issuance of the Series E preferred stock, as well as from the $20.0 million up-front payment made by Shell when we entered into our five-year research and development collaboration.
Interest Expense and Other, Net. Interest expense and other, net was $0.7 million in 2006, compared to $2.5 million in 2007, an increase of $1.8 million, or 250%. Interest expense and other, net in 2007 included the increase in the fair value of our Series D redeemable convertible preferred stock warrants, which resulted in $1.3 million of expense, interest expense on our outstanding financing obligations, and losses from foreign currency transactions.
Income Tax Benefit. The income tax benefit for 2006 and 2007 primarily consisted of benefit from reductions in deferred tax liabilities that had originated in a business acquisition, offset by foreign tax withheld at source on royalties earned overseas and other taxes attributable to foreign operations.
Liquidity and Capital Resources
Since inception, we have funded our operations through the sale of equity securities, borrowings under financing arrangements, collaborative research and development revenues, product sales and government grants. As of September 30, 2009, our cash, cash equivalents and marketable securities totaled $56.0 million. In addition, we have $0.7 million of restricted cash primarily related to letters of credit.
Operating Activities
We have historically experienced negative cash flow from operations as we continue to invest in our infrastructure and our technology platform, and expand our business. Our cash flows from operations will continue to be affected principally by the extent to which we increase our headcount, primarily in research and development, in order to grow our business. The timing of hiring of skilled research and development personnel in particular affects cash flows as there is a lag between the hiring of research and development personnel and the generation of collaboration or product revenues and cash flows from those personnel. Our primary source of cash flows from operating activities is cash receipts from our customers. Our largest uses of cash from operating activities are for employee related expenditures, rent payments, inventory purchases to support our revenue growth and non-payroll research and development costs, which include payments made to Maxygen in connection with our biofuels research and development collaboration with Shell. In light of the growth in market acceptance of our products and services to date, we do not expect to achieve profitability prior to at least 2011.
Our operating activities in the nine months ended September 30, 2009 used cash in the amount of $15.5 million, primarily as a result of our net loss of $15.1 million, decreases in deferred revenues of $4.4 million primarily as a result of continuing recognition of up-front exclusivity fees we received from
67
Table of Contents
Shell in 2007, and decreases in accounts payable and accrued liabilities of $3.2 million arising from the timing of payments to vendors. We also had net non-cash charges of $8.6 million, comprised primarily of $3.7 million in depreciation and amortization of property and equipment, $3.2 million in stock-based compensation expense, $0.7 million in amortization of intangible assets and $0.3 million related to the increase in the fair value of the redeemable convertible preferred stock warrants during the period.
Our operating activities used cash in the amount of $36.3 million in 2008, primarily due to our net loss of $45.1 million, an increase in inventories of $1.4 million, a decrease in a related party payable of $7.4 million, and offset by increases in accounts payable of $4.9 million and accrued liabilities of $5.3 million. These changes resulted primarily from the significant growth in our business, the timing of shipments and payments to vendors, including related parties, and our efforts to manage and monitor the balances of trade receivables. We also had net non-cash charges of $7.8 million, comprised primarily of $3.7 million in depreciation and amortization of property and equipment, $0.9 million in amortization of intangible assets, $3.5 million in stock-based compensation expense, and $0.5 million for amortization of debt discount.
Our operating activities used cash in the amount of $6.5 million in 2007, primarily due to our net loss of $39.0 million and an increase in accounts receivable of $3.1 million, partially offset by an increase in deferred revenues of $16.4 million, and an increase in accounts payable and accrued liabilities of $14.2 million. These changes resulted primarily from the significant growth in our business, the timing of shipments and payments to vendors, our efforts to manage and monitor the balances of trade receivables, and the increase in deferred revenues due to the timing of revenue recognition under our revenue recognition policy. We also had net non-cash charges of $6.3 million, comprised primarily of $2.1 million in depreciation and amortization of property and equipment, $1.2 million in amortization of intangible assets and deferred costs, $1.3 million in stock-based compensation expense, $1.3 million related to the increase in the fair value of the redeemable convertible preferred stock warrants, and $0.5 million expense related to preferred stock issued in exchange for services.
Based on our current level of operations and anticipated growth, we believe that our existing cash, cash equivalents and marketable securities, will provide adequate funds for ongoing operations, planned capital expenditures and working capital requirements for at least the next 12 months.
Investing Activities
In the nine months ended September 30, 2009, our investing activities used cash of $24.7 million, primarily for the net purchases of $18.4 million of marketable securities, and $6.5 million of capital expenditures. These capital expenditures consisted primarily of laboratory equipment purchases and leasehold improvements in our laboratories.
Our investing activities provided cash of $7.1 million in 2008, primarily from the net proceeds from the sale and maturities of marketable securities of $14.3 million, reduced by purchases of property and equipment of $8.5 million, and a decrease in restricted cash of $1.3 million. Restricted cash reduced by $0.8 million on payment of purchase consideration to a former shareholder of BioCatalytics and by $0.6 million on expiration of a letter of credit relating to a facility lease.
Our investing activities used cash of $39.2 million in 2007, primarily from net purchases of marketable securities of $28.5 million, the purchase of property and equipment of $8.2 million to support the growth in our business, the $1.3 million increase in restricted cash and net payments of $1.2 million for the BioCatalytics acquisition. The capital expenditures consisted primarily of laboratory equipment, computer and test equipment, and software purchases.
We expect our capital expenditures to be approximately $12.0 million for 2010. We are evaluating alternatives to manufacture biocatalysts at commercial scale. In the event we decide to build additional manufacturing facilities to manufacture biocatalysts at commercial scale, our capital expenditures will increase. We may be able to obtain government subsidies to offset all or a portion of the costs of building such facilities. In the future, we will continue to make laboratory equipment purchases to support our increasing research and development efforts and growth strategy.
68
Table of Contents
Financing Activities
In the nine months ended September 30, 2009, our financing activities provided $41.1 million in cash, primarily from the issuance and sale of 5.3 million shares of Series F preferred stock for $45.0 million, partially offset by $4.0 million in principal payments on our financing obligations. Subsequently, in November 2009, we issued 0.2 million additional shares of Series F preferred stock for $2.0 million.
Through our subsidiary, Jülich Fine Chemicals GmbH, we had a loan denominated in Euros with a German bank. The loan had a balance of 511,000 Euros (approximately $746,000 based on the Euro to U.S. dollar conversion at September 30, 2009) outstanding as of that date. In November 2009, in connection with the closure of our German operations, this balance was repaid.
Our financing activities used $3.9 million in cash during 2008, primarily from the $4.3 million in principal payments on our financing obligations, partially offset by $0.4 million in proceeds from the exercise of employee stock options.
Our financing activities provided cash of $68.4 million in 2007. The primary source of these funds was the issuance and sale of 6.1 million shares of Series E preferred stock and the exercise of warrants to purchase 0.4 million shares of Series D preferred stock, for an aggregate net consideration of $54.8 million from various investors. In September 2007, we borrowed a net amount of $14.8 million under the GE Capital Loan. The loan and security agreement for the GE Capital Loan, or the GE Capital Loan and Security Agreement, provides for $15.0 million in borrowings, is secured by substantially all of our assets with the exception of intellectual property, and bears interest at 9.4% per annum. The loan is to be repaid over 42 months from the date of funding, through monthly cash payments of principal and interest following six months of interest only payments. As of September 30, 2009, we had financing obligations of $9.5 million. The GE Capital Loan and Security Agreement contains a number of covenants that, among other things, restrict, subject to certain exceptions, our and our subsidiaries ability to:
| | incur additional debt or issue certain types of redeemable preferred stock; |
| | grant liens on our assets including our intellectual property; |
| | sell assets including our intellectual property; |
| | engage in mergers and acquisitions; |
| | declare or pay dividends to our stockholders; |
| | make investments, loans and advances; and |
| | amend our license agreement with Maxygen. |
The GE Capital Loan and Security Agreement also contains customary affirmative covenants including the requirement that we deliver certain financial statements, compliance certificates and capitalization tables to the lenders certified by our chief financial officer and provide the lenders with notice upon the occurrence of certain events. The GE Capital Loan and Security Agreement also contains customary events of default, the occurrence of which permit the lenders to declare all amounts outstanding under the GE Capital Loan and Security Agreement to be immediately due and payable. In addition, the lenders have the right to declare all amounts outstanding under the loan agreement to be immediately due and payable upon the occurrence of an event which has a material adverse effect on our business, assets or operations.
At December 31, 2008 and September 30, 2009, we were either in compliance with the covenants of the loan and security agreement, or obtained from the lenders waivers of noncompliance. In January 2008, GE, as agent for the lenders, waived certain events of default arising from our failure to timely deliver to GE monthly compliance certificates, financial statements and capitalization tables for each of the months from November 2007 to January 2008 and our annual operating plan for 2008. In addition, in August 2008, GE, as agent for the lenders, waived certain events of default arising from our failure to timely deliver to GE a copy of our registration statement on Form S-1 filed on April 14, 2008, monthly compliance certificates, financial statements and capitalization tables for each of the months from February to May 2008, and annual
69
Table of Contents
compliance certificates and audited financial statements for the fiscal years ended December 31, 2006 and December 31, 2007. The August 2008 waiver was provided in exchange for a waiver fee of $35,000, a general release of claims against GE and the other lenders and representations from us as to the absence of any other events of default under the GE Capital Loan and Security Agreement.
Contractual Obligations and Commitments
The following summarizes the future commitments arising from our contractual obligations at December 31, 2008 (in thousands):
| Total | 2009 | 2010 | 2011 | 2012 | 2013 and beyond |
|||||||||||||
| Loans payable(1) |
$ | 15,769 | $ | 6,351 | $ | 5,975 | $ | 3,443 | $ | | $ | | ||||||
| Capital leases(1) |
78 | 45 | 33 | | | | ||||||||||||
| Operating leases |
9,097 | 3,058 | 2,935 | 1,545 | 1,213 | 346 | ||||||||||||
| Total |
$ | 24,944 | $ | 9,454 | $ | 8,943 | $ | 4,988 | $ | 1,213 | $ | 346 | ||||||
| (1) | Amounts include interest on obligations. |
The table above reflects only payment obligations that are fixed and determinable. Our commitments for operating leases primarily relates to our leased facilities in Redwood City, California, and Jülich, Germany. In connection with the plans approved and committed to by our board of directors during 2009 to reduce our cost structure, we intend to close our facility in Jülich, Germany. In this regard, we anticipate we will incur lease termination costs of $0.4 million by December 31, 2009.
In February 2006, Jülich Fine Chemicals GmbH entered into a line of credit agreement with a German bank, to be used for both equipment purchases and working capital requirements in the amount of $184,000. Prior to its acquisition, Jülich Fine Chemicals GmbH had entered a line of credit with a German bank, to be used for both equipment purchases and working capital requirements. No balances were outstanding on these lines of credit at December 31, 2008, and they were cancelled during 2009.
Off-Balance Sheet Arrangements
As of September 30, 2009, we have no off-balance sheet arrangements as defined in Item 303(a)(4) of Regulation S-K as promulgated by the SEC.
Recent Accounting Pronouncements
In June 2009, the FASB issued Statement of Financial Accounting Standard, or SFAS, No. 168, The FASB Accounting Standards Codification and the Hierarchy of Generally Accepted Accounting Principles A Replacement of FASB Statement No. 162, or SFAS 168. SFAS 168, which is incorporated in Accounting Standards Codification, or ASC, Topic 105, Generally Accepted Accounting Principles, identifies the ASC as the authoritative source of generally accepted accounting principles in the United States. Rules and interpretive releases of the SEC under federal securities laws are also sources of authoritative generally accepted accounting principles for SEC registrants. We adopted the provisions of the authoritative accounting guidance for the interim reporting period ended September 30, 2009 and included references to the ASC within our consolidated financial statements. The adoption had no impact on our consolidated results of operations or financial position.
In September 2006, the FASB issued SFAS No. 157, Fair Value Measurements, or SFAS 157, which is incorporated in ASC Topic 820, Fair Value Measurements and Disclosures. SFAS 157 defines fair value, establishes a framework for measuring fair value and requires additional disclosures about fair value measurements. In February 2008, the FASB issued FASB Staff Position, or FSP, FAS 157-1, Application of FASB Statement No. 157 to FASB Statement No. 13 and Other Pronouncements that Address Fair Value Measurements for Purpose of Lease Classification or Measurement under Statement 13, which is
70
Table of Contents
incorporated in ASC Topic 820, which amends SFAS 157 to exclude accounting pronouncements that address fair value measurements for purposes of lease classification or measurement under SFAS No. 13, Accounting for Leases. In February 2008, the FASB also issued FSP SFAS No. 157-2, Effective Date of FASB Statement No. 157, which is incorporated in ASC Topic 820, which delays the effective date of SFAS 157 until the first quarter of 2009 for all non-financial assets and non-financial liabilities, except for items that are recognized or disclosed at fair value in the consolidated financial statements on a recurring basis, at least annually. SFAS 157 does not require any new fair value measurements but rather eliminates inconsistencies in guidance found in various prior accounting pronouncements. In April 2009, the FASB further issued FSP SFAS No. 157-4, Determining Fair Value When the Volume and Level of Activity for the Asset or Liability Have Significantly Decreased and Identifying Transactions That Are Not Orderly, or FSP SFAS 157-4, which is incorporated in ASC Topic 820. FSP SFAS 157-4 is effective for interim and annual periods ending after June 15, 2009, with early adoption permitted. We adopted SFAS 157 and such adoption did not have a significant impact on our consolidated financial statements.
In December 2007, the FASB ratified EITF Issue No. 07-1, Accounting for Collaborative Agreements, or EITF 07-1, which defines collaborative agreements as contractual arrangements that involve a joint operating activity. EITF 07-1, which is incorporated in ASC Topic 808, Collaborative Agreements, states that these arrangements involve two or more parties who are both active participants in the activity and that are exposed to significant risks and rewards dependent on the commercial success of the activity. EITF 07-1 provides that a company should report the effects of adoption as a change in accounting principle through retrospective application to all periods. Furthermore, it requires the parties to determine who is the principal party of the arrangement, and therefore which party must report the revenues and expenses under the collaboration arrangement, as well as specific additional disclosures in the parties financial statements. EITF 07-1 is effective for periods beginning after December 15, 2008. We adopted EITF 07-1 on January 1, 2009. The adoption did not have a significant effect on our consolidated results of operations or financial position.
In May 2009, the FASB issued SFAS No. 165, Subsequent Events, or SFAS 165, which is incorporated in ASC Topic 855, Subsequent Events. The standard establishes general standards of accounting for and disclosure of events that occur after the balance sheet date but before financial statements are issued or are available to be issued. Although there is new terminology, the standard is based on the same principles as those that are currently exist in the auditing standards. The standard, which includes a new required disclosure of the date through which an entity has evaluated subsequent events, is effective for interim or annual periods ending after June 15, 2009. We adopted the provisions of this authoritative guidance in the nine month period ended September 30, 2009. The adoption had no impact on our consolidated results of operations or financial position.
In October 2009, the FASB issued Accounting Standards Update, or ASU, 2009-13, which amends ASC Topic 605, Revenue Recognition, to require companies to allocate revenues in multiple-element arrangements based on an elements estimated selling price if vendor-specific or other third-party evidence of value is not available. ASU 2009-13 is effective beginning January 1, 2011. Earlier application is permitted. We are currently evaluating both the timing and the impact of the pending adoption of the ASU on our consolidated financial statements.
Quantitative and Qualitative Disclosures about Market Risk
Interest Rate Sensitivity
We had unrestricted cash, cash equivalents and marketable securities totaling $84.1 million, $37.1 million and $56.0 million at December 31, 2007 and 2008 and September 30, 2009, respectively. These amounts were invested primarily in money market funds, corporate debt obligations, U.S. government-sponsored enterprise securities, and U.S. Treasury securities and are held for working capital purposes. We do not enter into investments for trading or speculative purposes. We believe we do not have material exposure to changes in fair value as a result of changes in interest rates. Declines in interest rates, however,
71
Table of Contents
will reduce future investment income. If overall interest rates fell by 10% in 2008 and the nine months ended September 30, 2009, our interest income would have declined by approximately $0.1 million and $11,000, respectively, assuming consistent investment levels.
The terms of our GE Capital Loan provide for a fixed rate of interest, and therefore is not subject to fluctuations in market interest rates.
Foreign Currency Risk
Our operations include manufacturing and sales activities in the United States, Austria, France, Germany, Italy, Japan and India, as well as research activities in countries outside the United States, including Singapore, Germany and Hungary. As we expand internationally, our results of operations and cash flows will become increasingly subject to fluctuations due to changes in foreign currency exchange rates. For example, we purchase materials for, and pay employees at, our research facility in Singapore in Singapore dollars. In addition, we purchase products for resale in the United States from foreign companies and have agreed to pay them in currencies other than the U.S. dollar. As a result, our expenses and cash flows are subject to fluctuations due to changes in foreign currency exchange rates. In periods when the U.S. dollar declines in value as compared to the foreign currencies in which we incur expenses, our foreign-currency based expenses increase when translated into U.S. dollars. Although it is possible to do so, we have not hedged our foreign currency since the exposure has not been material to our historical operating results. Although substantially all of our sales are denominated in U.S. dollars, future fluctuations in the value of the U.S. dollar may affect the price competitiveness of our products outside the United States. The effect of a 10% adverse change in exchange rates on foreign denominated receivables as of December 31, 2008 and September 30, 2009, would have been a $0.3 million and $0.4 million foreign exchange loss recognized as a component of interest expense and other, net in our consolidated statement of operations. We may consider hedging our foreign currency as we continue to expand internationally.
Controls and Procedures
We have not performed an evaluation of our internal control over financial reporting, such as required by Section 404 of the Sarbanes-Oxley Act, nor have we engaged our independent registered public accounting firm to perform an audit of our internal control over financial reporting as of any balance sheet date or for any period reported in our financial statements. Had we performed such an evaluation or had our independent registered public accounting firm performed an audit of our internal control over financial reporting, control deficiencies, including material weaknesses and significant deficiencies, in addition to those discussed below, may have been identified.
Solely in connection with the audit of our consolidated financial statements for 2005, 2006 and 2007, we and our independent registered public accounting firm identified a material weakness in our internal control over financial reporting. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of a companys annual or interim financial statements will not be prevented or detected on a timely basis.
The material weakness comprised (i) our lack of policies and procedures, with the associated internal controls, to appropriately address complex, non-routine transactions and (ii) the lack of a sufficient number of qualified personnel to timely account for such transactions in accordance with U.S. generally accepted accounting principles.
We and our independent registered public accounting firm identified the following issues during the audit, which collectively gave rise to the conclusion that we had an underlying material weakness:
| | inadequate policies to address the increasingly complex revenue arrangements that we enter into; |
| | failure to correctly and timely identify all data required to evaluate the accounting impact of stock option grants and to recognize all requirements under applicable accounting standards; |
72
Table of Contents
| | accounting issues that arose in connection with two acquisitions; |
| | improper recording of foreign currency cumulative translation adjustments; and |
| | failure to effectively track and value inventory. |
These deficiencies in the design and operation of our internal controls resulted in the recording of numerous audit adjustments, and significantly delayed our financial statement close process, for the three year period ended December 31, 2007.
Solely in connection with the audit of our consolidated financial statements for 2008, we and our independent registered public accounting firm identified a material weakness and two significant deficiencies in our internal control over financial reporting. The material weakness related to an inadequately designed process to analyze and reconcile certain accounts and the failure of supervisors or business unit managers to review the analysis prepared for certain accounts. We and our independent registered public accounting firm identified the following issues during the audit, which collectively gave rise to the conclusion that we had an underlying material weakness:
| | duplicative accrual relating to legal expenses in our accounting records; |
| | incorrect inputs relating to the Black-Scholes calculation of fair value of non-employee stock options and warrants; |
| | under accrual of expenses for tax consulting work; |
| | incorrect input of costs eligible for reimbursement under a license agreement; |
| | errors in the valuation of inventory; and |
| | failure to accrue penalties associated with foreign filing requirements. |
We and our independent registered public accounting firm also identified two significant deficiencies in our internal control over financial reporting relating to (i) misapplication of U.S. generally accepted accounting principles and (ii) an ineffective contract compliance process. The material weakness and significant deficiencies resulted in the recording of numerous audit adjustments.
Notwithstanding the material weaknesses described above, we have performed additional analyses and other procedures to enable management to conclude that our consolidated financial statements included in this filing were prepared in accordance with U.S. generally accepted accounting principles.
We have not yet been able to remediate the deficiencies identified in these audits. However, we have taken numerous steps and plan to take additional significant steps intended to address the underlying causes of the material weaknesses and significant deficiencies in the immediate future, primarily through the development and implementation of formal policies, improved processes and documented procedures, the retention of third-party experts and contractors, and the hiring of additional accounting and finance personnel with technical accounting, inventory accounting and financial reporting experience. The actions that we have taken are subject to ongoing senior management review, as well as audit committee oversight. We do not know the specific timeframe needed to remediate all of the control deficiencies underlying these material weaknesses and significant deficiencies. In addition, we may incur significant incremental costs associated with this remediation, primarily due to the procurement, implementation and validation of robust accounting and financial reporting systems, the hiring of additional finance and accounting personnel and the continued use of third-party experts and contractors to assist us.
If we fail to enhance our internal control over financial reporting to meet the demands that will be placed upon us as a public company, including the requirements of the Sarbanes-Oxley Act, we may be unable to accurately report our financial results, or report them within the timeframes required by law or exchange regulations. We will be required to meet the requirements of Section 404 of the Sarbanes-Oxley Act beginning with our fiscal year ending December 31, 2011.
73
Table of Contents
Company Overview
Our proprietary technology platform enables the creation of optimized biocatalysts that make existing industrial processes faster, cleaner and more efficient than current methods and has the potential to make new industrial processes possible at commercial scale. We have commercialized our biocatalysts in the pharmaceutical industry and are developing biocatalysts for use in producing advanced biofuels under a multi-year research and development collaboration with Shell. We are also using our technology platform to pursue biocatalyst-enabled solutions in other bioindustrial markets, including carbon management, water treatment and chemicals.
Biocatalysts are enzymes or microbes that initiate or accelerate chemical reactions. Manufacturers have historically used naturally occurring biocatalysts to produce many goods used in everyday life. However, inherent limitations in naturally occurring biocatalysts have restricted their commercial use. Our proprietary technology platform is able to overcome many of these limitations, allowing us to evolve and optimize biocatalysts to perform specific and desired chemical reactions at commercial scale.
We have focused our biocatalyst development efforts on large and rapidly growing markets, including pharmaceuticals and advanced biofuels. We have enabled biocatalyst-based drug manufacturing processes at commercial scale and have delivered biocatalysts, intermediates and active pharmaceutical ingredients, or APIs, to some of the worlds leading pharmaceutical companies, including Dr. Reddys Laboratories Ltd., Merck & Co., Inc., Pfizer Inc. and Ranbaxy Laboratories Limited. In our collaboration with Shell, we are developing biocatalysts for use in producing advanced biofuels from renewable sources of non-food plant materials, known as cellulosic biomass.
We were incorporated in Delaware in January 2002 as a wholly-owned subsidiary of Maxygen, Inc. We commenced independent operations in March 2002, after licensing from Maxygen core enabling technology. As of September 30, 2009, Maxygen beneficially owned approximately 21.6% of our common stock. Our other investors include industry leaders such as Shell, Chevron Corporation, Pfizer and The General Electric Company.
Biocatalyst Opportunity
Biocatalyst-enabled manufacturing processes may address a number of the drawbacks of conventional chemistry-based manufacturing. For example, unlike most chemistry-based manufacturing processes, biocatalysts can operate at or near room temperature and pressure, and often use manufacturing equipment that is less complex and expensive to build and operate. Biocatalyst-enabled processes can create products with the same or higher quality as chemistry-based manufacturing processes, while reducing risks associated with extreme manufacturing environments and without generating the high volumes of waste, some of it hazardous to health and the environment, typically associated with conventional chemistry-based manufacturing processes.
In addition, due to concerns about the environment and the scarcity and security of supply of petroleum, there is an increasing interest in using cellulosic biomass as the feedstock for a variety of products, including advanced biofuels and other chemicals as a replacement for petroleum. To date, conventional chemistry-based manufacturing approaches have not resulted in commercially viable processes for the conversion of cellulosic biomass to biofuels and other products. Biocatalysts have the potential to enable processes for the development of products, such as cellulose-derived biofuels, that cannot currently be manufactured using alternative techniques.
Despite their potentially significant advantages, biocatalysts have not achieved their full potential in industrial applications. Naturally occurring biocatalysts are often not stable enough to be used in industrial
74
Table of Contents
settings, where conditions may differ significantly from those in the biocatalysts natural environments. The activity and productivity of these biocatalysts is often too limited to be cost-effective in commercial scale manufacturing. In addition, the activity of natural biocatalysts is typically inhibited by the end product of the reactions they facilitate. This characteristic of natural biocatalysts, which is referred to as product inhibition, results in limited product yields in industrial settings. Moreover, for certain industrial applications, there are no known naturally occurring biocatalysts that catalyze the desired reaction.
Due to these limitations, other companies and researchers have tried to improve the performance of naturally occurring biocatalysts by directing their evolution through biotechnology techniques such as the random mutation of genes. However, to date, these techniques have had only limited success for a number of reasons. For example, random mutations of genes often result in decreased, not improved, performance and these alternative biotechnology techniques cannot effectively remove accumulated detrimental mutations. The end result is often an evolved biocatalyst with activity that reaches a plateau at a level that is insufficient for a commercial process. We believe there is a significant opportunity for novel technologies that can address the limitations of other biotechnology techniques and can substantially enhance the performance of biocatalysts in industrial settings.
Our Platform Technology
We believe that our proprietary technology platform can transform the industrial application of biocatalysts by improving their commercially relevant characteristics, such as stability, activity, product yield and tolerance to industrial conditions, while reducing product inhibition. In addition, our technology platform allows us to develop and optimize biocatalysts much more rapidly than is currently possible with alternative methods. Perhaps most importantly, we have demonstrated that our technology platform can enable the manufacture of products cost-effectively, at commercial scale and with significantly reduced environmental impact relative to conventional manufacturing processes.
Our proprietary technology platform uses advanced biotechnology methods, bioinformatics and years of accumulated know-how to significantly expedite the process of developing optimized biocatalysts. Key components of our technology platform include gene shuffling, whole genome shuffling, multiplexed gene SOEing, and proprietary bioinformatic software tools that allow us to identify and quantify the potential value of beneficial mutations and avoid detrimental mutations.
Application in Pharmaceuticals
In the pharmaceutical market, our technology platform has significantly improved commercial scale drug manufacturing processes. Our customers have benefited from our processes and products through:
| | reduced costs, including capital and operating costs; |
| | simplified production processes; |
| | decreased environmental impact; and |
| | increased efficiency and product yield. |
For example, we have used our technology platform to develop four biocatalysts that enabled significant improvements in the manufacturing processes for key intermediates used in the production of atorvastatin, which is the active pharmaceutical ingredient, or API, in Lipitor, the worlds best-selling prescription drug. Manufacturers have historically used a complex, expensive, capital intensive and hazardous chemistry-based process to produce these intermediates, called ATS-5 and ATS-8. As a result, they have long sought alternate ways to make the drug, including through biocatalysts-enabled processes. However, none of the naturally occurring enzymes that we tested showed the required activity and stability necessary for their manufacture. We first developed a new two step process using three optimized
75
Table of Contents
biocatalysts for the production of ATS-5, which Pfizer purchases as their starting material to make atorvastatin. Using our technology platform, we:
| | significantly improved the activity and stability of all three biocatalysts, including increasing the performance of one of them, which previously showed only 0.25% of the required activity and stability, by approximately 4,000 times; |
| | eliminated the need for a costly purification step due to the high purity of the product that is generated by our process, resulting in additional cost savings; and |
| | obtained higher yields than the alternative conventional chemical processes for ATS-5. |
We received a Presidential Green Chemistry Challenge Award from the United States Environmental Protection Agency for the development of our biocatalytic manufacturing process for ATS-5.
The next key isolated intermediate for atorvastatin is ATS-8, which we supply to manufacturers of generic atorvastatin. We replaced the second of three steps in the manufacture of ATS-8 with a biocatalytic reaction. Using our technology platform, we:
| | significantly improved the activity and stability of the fourth biocatalyst to enable the process; |
| | replaced a step that previously required temperatures below -70 degrees Celsius and used hazardous agents with a benign biocatalytic step that runs at or near room temperature, eliminating the need for expensive and energy intensive cryogenic equipment; and |
| | obtained higher purity product, eliminating the need for a yield-reducing ATS-8 purification step. |
For both ATS-5 and ATS-8, we greatly reduced the waste generated by the conventional chemistry-based processes and generated a biodegradable waste from two of the steps.
Application in Biofuels and Other Bioindustrial Markets
We are also using our technology platform to develop biocatalysts for use in producing advanced biofuels that currently cannot be manufactured cost-effectively at commercial scale. Advanced biofuels are liquid transportation fuels derived from non-food biomass and which meet certain minimum carbon reduction criteria. As part of our research and development collaboration with Shell, we have used our technology platform to:
| | improve our cellulase biocatalysts to increase their production of fermentable sugars from cellulosic biomass; |
| | enable our cellulase biocatalysts to operate in a wider range of operating conditions; and |
| | develop a microbe that converts cellulosic biomass-derived sugar to diesel fuel, which is secreted out of the cell. |
In addition, we are using our technology platform to improve the yields from ethanol-producing yeast.
We are also using our technology platform to develop biocatalysts to optimize the process of removing carbon dioxide from flue gases in coal-fired energy generation plants. Our biocatalysts improve the effectiveness of a range of solvents, including amine solvents, which is one of the leading potential technologies to remove carbon dioxide from flue gas. In the laboratory, these biocatalysts have exhibited increased tolerance for flue stack-type operating conditions, though not yet at target commercial levels. We also intend to use our technology platform to pursue biocatalyst solutions in other bioindustrial markets, including water treatment and chemicals.
76
Table of Contents
Our Business Model
Our business model allows us to simultaneously pursue multiple commercial opportunities across a number of major markets. Our business model has resulted in a diversified revenue stream that is predictable over the near term and has a significant growth potential, while allowing us to share risk with and leverage the capabilities of our collaborators. Our business model includes the following key elements:
Targeting Multiple Major and Growing Markets. We currently use our technology platform to produce biocatalysts that are used at commercial scale in the pharmaceutical market. Through our collaboration with Shell, we are developing biocatalysts for use in producing commercially viable biofuels from cellulosic biomass. We also believe that we can use our technology platform to deliver biocatalyst- enabled solutions to other bioindustrial markets, including carbon management, water treatment and chemicals.
Capital-Efficient Collaborations with Industry Leaders. We have adopted a business model that leverages our collaborators engineering, manufacturing and commercial expertise, their distribution infrastructure and their ability to fund commercial scale production facilities. For instance, in the pharmaceuticals market, our supply relationship with Arch enables us to bring intermediates and/or APIs for branded pharmaceutical products to market with very limited additional capital. In addition, if we are able to develop biocatalysts that enable the commercial production of biofuels derived from cellulosic biomass and Shell decides to commercialize products based on this technology, we would need to rely on Shell, or other parties selected by Shell, to design and build the commercial scale fuel production facilities and to distribute the final fuel product.
Diversified Revenue Base. We are generating a revenue stream that is diversified across distinct industries, which should mitigate our exposure to cyclical downturns or fluctuations in any one market. In 2008, our revenues were derived from the pharmaceuticals and biofuels markets, and consisted primarily of collaborative research and development revenues and product sales. We are pursuing biocatalyst-enabled solutions in other bioindustrial markets, including carbon management, water treatment and chemicals, that if successful, will allow us to further diversify our revenues.
Visible and Predictable Revenues. Based on our existing arrangements, we believe that the revenues from both our biofuels and pharmaceutical businesses should be predictable over the near term. We receive bi-monthly payments from Shell that are based on the number of funded FTEs that work on our research collaboration with Shell. The number of funded FTEs that work on the program, and the payments from Shell for these FTEs, are specified in our collaborative research agreement, subject to Shells ability to increase or reduce the number of FTEs under certain conditions over time. Because we allow our pharmaceutical customers to achieve significant cost savings in their manufacturing processes, historically they have continued using our biocatalysts once they have begun using our biocatalyst-enabled process.
Our Strategy
Our objective is to be the leading provider of optimized biocatalyst-enabled solutions across a wide range of industries. Key elements of our strategy are as follows:
Become a leading biocatalyst supplier to the advanced biofuels market. Our primary development efforts are focused on producing biocatalysts that can enable Shell to become a global leader in the advanced biofuels market. We continue to build upon our milestone-driven, multi-year collaboration with Shell as we advance our efforts to produce biofuels from cellulosic biomass cost-effectively at commercial scale. Because of our success to date, Shell has expanded our research and development collaboration twice, which we believe positions us to be a key contributor to their overall biofuels strategy.
Expand into new bioindustrial markets. We are actively pursuing opportunities in other bioindustrial markets, including through self-funded research in carbon management and the pursuit of funded collaborations in carbon management, water treatment and chemicals. We have the right to use the
77
Table of Contents
intellectual property developed in our collaboration with Shell in fields outside of fuels and related products. We intend to leverage this and other intellectual property and our technology platform to develop products in our other target markets.
Continue growing our pharmaceutical business. We intend to pursue new collaborations in the pharmaceutical industry to integrate our products and services more deeply into drug development and manufacturing processes for clinical stage and commercially approved pharmaceutical products. As part of that effort, we will continue to aggressively market our Codex Biocatalyst Panels to pharmaceutical companies to demonstrate the capabilities of our technology platform.
Secure access to additional production capacity. To increase our biocatalyst manufacturing capacity and establish secondary supply sources, we are working to establish long-term supply contracts with contract manufacturers and are evaluating whether to invest in our own manufacturing capabilities. We may also opportunistically seek to secure specialty manufacturing assets and expand existing relationships for the supply of our biocatalysts and key pharmaceutical APIs and intermediates. For example, in August 2008, we entered into an expanded supply relationship with Arch for the manufacture of intermediates and APIs for specified pharmaceutical products.
Expand our business and technology platform through the addition of new technologies, products or businesses. In the past, we have expanded our business by acquiring companies with synergistic business plans and licensing new technology. We will continue to evaluate opportunities to acquire or license new technologies, products or businesses that complement or expand our capabilities, including in the carbon management, water treatment and chemical markets. In addition, we intend to continue to advance our technology platform by investing in our research and development capabilities to allow us to more rapidly identify and develop products and pursue new market opportunities.
Our Pharmaceutical Business
Our Opportunity in the Pharmaceutical Market
The pharmaceutical industry represents a significant market opportunity for us. In 2008, according to IMS Health, global spending on prescription drugs was $773 billion. Pharmaceutical companies are now under significant competitive pressure both to reduce costs and increase the speed to market for their products. To meet these pressures, they are seeking manufacturing processes for their new products and existing drugs that reduce overall costs, simplify production and increase efficiency and product yield, while not affecting drug safety and efficacy. In addition, for products whose patents have expired, the importance of cost reduction is even higher, as the pharmaceutical manufacturers which had developed those patent-protected drugs, known as innovators, compete with generics manufacturers.
The pharmaceutical product lifecycle begins with the discovery of new chemical entities and continues through preclinical and clinical development, product launch and, ultimately, patent expiration and the transition from branded to generic products. As innovators develop, produce and then market products, manufacturing priorities and processes evolve. Historically, innovators have focused on production cost reduction in the later stages of clinical development but have been reluctant to make process changes after a product has been launched. However, as pressures to reduce costs have increased, innovators have pursued cost reduction measures much earlier in the pharmaceutical product lifecycle and are increasingly looking for opportunities to improve their operating margins, including making manufacturing process changes for marketed products if these changes can result in significant cost reductions. As a result, innovators are investing in new technologies to improve their manufacturing productivity and efficiency or outsourcing the manufacture of their intermediates and APIs.
Another strategy innovators can use to reduce costs is to adopt manufacturing processes that obviate the need for costly purification of their intermediates or APIs. For example, the chemical structure of many small molecule drugs has two or more configurations, similar to a persons left and right hands. While the
78
Table of Contents
two or more configurations have the same chemical structures, there can be differences in their therapeutic safety and efficacy profiles. To avoid developing a drug containing configurations with detrimental effects, pharmaceutical companies are increasingly seeking to introduce new drugs containing only the desired configuration. Manufacturing the pure configurations via conventional chemistry-based processes is rarely possible in a cost-effective manner at commercial scale. These conventional chemistry-based processes typically require late-stage purification steps that reduce product yield and can significantly increase costs. Because of the high costs associated with these purification steps, significant opportunities exist for alternatives that can produce pure configurations using more efficient and less costly methods.
Generics manufacturers are also increasingly pursuing opportunities to reduce costs. The rise in patent expirations, as well as support by some governments for lower-cost alternatives to branded drugs, have led to strong growth in the generics industry. According to Datamonitor, generic competition is expected to eliminate $117 billion from top innovators worldwide sales between 2008 and 2014 as approximately three dozen drugs are expected to lose patent protection. In addition, according to IMS Health, generics products account for 64% of the total pharmaceutical market in the United States. However, because generics manufacturers compete primarily on price, they are even more cost sensitive than innovators. Lower manufacturing costs for intermediates and APIs is the key factor that helps generics companies compete and win market share. Prior to the expiration of patents on a branded drug, generics manufacturers also have significant opportunities to commercialize the generic equivalents of branded drugs in the markets which do not provide effective patent protection.
Our Solution for the Pharmaceutical Market
Our technology platform enables us to deliver solutions to our customers in the pharmaceutical market by developing and delivering optimized biocatalysts that perform chemical transformations at a lower cost, and improve the efficiency and productivity of manufacturing processes. We provide value throughout the pharmaceutical product lifecycle. Our technology platform allows us to provide benefits to our customers in a number of ways, including:
| | reducing the use of raw materials and intermediate products; |
| | improving product yield; |
| | using water as a primary solvent; |
| | performing reactions at or near room temperature and pressure; |
| | eliminating the need for certain costly manufacturing equipment; |
| | reducing energy requirements; |
| | reducing the need for late-stage purification steps; |
| | eliminating multiple steps in the manufacturing process; and |
| | eliminating hazardous inputs and harmful emission by-products. |
Early in the product lifecycle, customers can use our services to achieve speed to market and to reduce manufacturing costs. If an innovator incorporates our products or processes into an FDA-approved product, we expect the innovator to continue to use these products or processes for the patent life of the approved drug.
After a product is launched, customers also use our services to reduce manufacturing costs. At this stage, changes in the manufacturing process originally approved by the FDA may require additional review. Typically, pharmaceutical companies will only seek FDA approval for a manufacturing change if there are substantial cost savings associated with the change. We believe that the cost savings associated with our products may lead our customers to change their manufacturing processes for approved
79
Table of Contents
products and, if necessary, seek FDA approval of the new processes which incorporate our biocatalysts. Moreover, we believe these cost savings are attractive to generics manufacturers, who compete primarily on price.
Products and Services
Codex Biocatalyst Panels. We sell Codex Biocatalyst Panels to customers who are engaged in both drug development and the marketing of approved drugs to allow them to screen and identify possible biocatalytic manufacturing processes for their drug candidates and their marketed products. Our Codex Biocatalyst Panels are plates embedded with genetically diverse variants of our proprietary biocatalysts, which allow our customers to determine whether a biocatalyst produces a desired activity that is applicable to a particular process.
For compounds that are in development, our Codex Biocatalyst Panels:
| | allow innovators to rapidly and inexpensively screen and identify possible biocatalytic manufacturing processes for many of their drug candidates in-house, without the risks of disclosing the composition of their proprietary molecules before they have received patent protection; and |
| | generate data that we can use to rapidly optimize biocatalysts for a particular reaction, if necessary, reducing the time required to generate a manufacturing process capable of supporting clinical trials with inexpensively produced, pure drugs. |
We believe that our Codex Biocatalyst Panels have helped us build early and broad awareness of the power and utility of our technology platform, and will increasingly lead to sales of our biocatalyst optimization services and biocatalysts, as well as intermediates and APIs made using our biocatalysts. We currently have over ten customers for our panels, including leading pharmaceutical companies such as F. Hoffman-La Roche Ltd., GlaxoSmithKline plc, Merck, Novartis and Pfizer. If our customers incorporate a biocatalytic manufacturing process early in a products lifecycle, they can reduce their manufacturing costs throughout that lifecycle, while we, in turn, could realize a long term revenue stream resulting from the use of our biocatalysts during that time. In addition, our Codex Biocatalyst Panels are increasingly used by our customers to evaluate the feasibility of changing the manufacturing process for their marketed products to a biocatalyst-enabled process.
Biocatalyst screening services. If a customer prefers, rather than subscribing to our Codex Biocatalyst Panels to use for their own screening, they can send us their materials to test against our existing libraries of biocatalysts. If we detect desired activity in a specific biocatalyst, we can supply the customer with this biocatalyst or perform optimization services to improve the performance of the biocatalyst.
Our screening services:
| | allow innovators to rapidly and inexpensively screen and identify possible biocatalytic manufacturing processes through access to our extensive biocatalyst libraries; and |
| | generate data that we can use to rapidly optimize biocatalysts for a particular reaction, if necessary, reducing the time required to generate a manufacturing process capable of supporting the customers particular needs, ranging from small quantities for clinical trials to full commercial production, in all cases providing inexpensively produced, pure drugs. |
We have provided screening services to numerous innovator and generic pharmaceutical manufacturers.
Biocatalyst optimization services. We work with our customers throughout the pharmaceutical product lifecycle to customize proprietary biocatalysts, resulting in optimized biocatalysts that have been evolved specifically to perform a desired process according to a highly selective set of specifications.
80
Table of Contents
Our biocatalyst optimization services:
| | allow innovators to improve the manufacturing process as their drug candidates progress through preclinical and clinical development, deferring or reducing the need for significant manufacturing investment until the likelihood of commercial success is more certain; and |
| | enable manufacturing processes that are highly efficient, inexpensive, require relatively little energy, reduce the need for hazardous reagents, and reduce waste. For example, our activities with Pfizer have included developing an optimized biocatalytic manufacturing process for a key intermediate that eliminates three chemical steps. |
Biocatalysts. We supply varying quantities of our proprietary biocatalysts to pharmaceutical companies, from small to moderate quantities while they are optimizing their production processes, to larger quantities during later-stage clinical development and commercial scale drug production.
Our biocatalysts:
| | enable innovators to manufacture products more efficiently during preclinical and clinical development using optimized biocatalytic processes, with relatively low investment; |
| | eliminate the need for innovators to invest in the development of complex chemical synthesis routes during the development stage; |
| | allow innovators to achieve higher product purity during the development stage prior to investing in expensive late-stage clinical trials; |
| | reduce the risk of adverse effects arising from product impurities; |
| | allow the removal of entire steps from synthetic chemical production routes during commercial scale production, reducing raw material costs, energy requirements and the need for capital expenditures; and |
| | decrease the manufacturing costs for our customers. |
Intermediates and APIs. We can supply our customers intermediates and APIs made using our biocatalysts throughout the drug lifecycle.
Our supply of intermediates has the following uses and benefits:
| | lowers capital investment for innovators through outsourcing of manufacturing; and |
| | provides a source of less expensive, more pure products to innovator and generics manufacturers. |
In the innovator market, we are currently supplying Pfizer with an intermediate in the manufacture of Lipitor. We have also developed biocatalysts for use in the manufacture of certain generic intermediates and APIs by various companies, including Arch and Teva Pharmaceutical Industries Ltd., or Teva. In addition, we have launched and are marketing several new intermediates and APIs for the generic equivalents of branded pharmaceutical products, including Singulair and Cymbalta, for sale in markets where innovators have not sought patent protection for their products and intend to sell these same intermediates and APIs for use in markets where innovators have sought patent protection when the patent protection for each product expires.
81
Table of Contents
Our Biofuels Business
Industry Overview Need for Petroleum Replacement
The worlds economy is heavily dependent on petroleum. However, economic, political and environmental concerns surrounding petroleum have increased the desire to find renewable alternatives to this limited commodity.
| | Increasing demand for petroleum. While the United States, Europe and Japan have historically been the major consumers of petroleum, developing economies such as India and China are experiencing tremendous levels of economic growth. In 2008, China and India alone saw GDP growth rates estimated at 9.0% and 7.4%, respectively. This economic growth has created new sources of demand for petroleum, with China and Indias combined share growing from 10% of the worlds total energy consumption in 1990 to 19% in 2006 and forecasted to grow to 28% of the worlds energy consumption by 2030. |
| | Dependence on imported petroleum. According to the U.S. Energy Information Administration, or EIA, in 2008, the top five net oil exporting countries in the world were Saudi Arabia, Russia, the United Arab Emirates, Iran and Kuwait. The political and economic instability in some of these countries and their surrounding regions adds further uncertainty to the supply of oil. As a result, countries that have been net importers of oil are beginning to pursue approaches that provide for greater independence from these suppliers. |
| | Expense of developing new petroleum reserves. The cost to replace known reserves is increasing significantly. Petroleum companies are now developing fields in the deep waters of the Gulf of Mexico and in the tar sands in Canada that previously would have not been economically attractive to exploit. |
| | Rising and volatile petroleum prices. According to the EIA, worldwide petroleum prices in dollars have risen 213% and fluctuated significantly over the last ten years, from $25.01 per barrel at the beginning of December 1999, to $78.39 per barrel at the start of December 2009. In addition to rising prices, petroleum pricing has been highly volatile with significant price spikes over time, including prices reaching a record high of $145.31 per barrel in July 2008. |
| | Limited supply of petroleum. Growth in demand for petroleum has outpaced growth in supply. The supply growth has come mostly from non-OPEC producing countries. However, this growth is expected to flatten. While OPEC producing countries may have the reserves, political instability in these regions has hindered their ability to increase production levels. |
| | Environmental concerns and regulatory initiatives. Environmental concerns over the by-products of petroleum consumption, including greenhouse gas emissions, have led to a global search for alternative solutions to the worlds growing fuel needs. For example, the American Clean Energy and Security Act, otherwise known as the Waxman-Markey climate and energy bill, seeks to mandate, among other things, emission cuts and permits for emissions in certain regulated industries. In addition, in December 2009, government representatives from all over the world convened at the United Nations Framework Convention on Climate Change in Copenhagen, Denmark with the goal of creating a global climate change protocol to follow the Kyoto Protocol. |
Industry Challenges and Opportunities
According to the EIA, global petroleum demand in 2008 was 86 million barrels per day; historically 45% of this demand has been refined into liquid transportation fuels for use in automobiles. There is enormous potential to replace a substantial portion of petroleum-based liquid transportation fuels with high-quality, energy-rich fuels produced through biocatalyst-enabled transformation of renewable cellulosic biomass sources. For instance, the U.S. Congress passed the Energy Independence and Security
82
Table of Contents
Act of 2007, an alternative fuels mandate that calls for 13 billion gallons of liquid transportation fuels sold in 2010 to come from alternative sources, including biofuels, a mandate that grows to 36 billion gallons by 2022. This mandate requires that of the 36 billion gallons, 21 billion gallons must be advanced biofuels. First generation biofuels manufacturers use biocatalysts to produce biofuels from food-based biomass and plant oils, such as ethanol and biodiesel. However, fuels produced from these sources do not provide an optimal solution to the petroleum dependence problem for a number of reasons, including:
| | high exposure to rising commodity and energy prices; |
| | potential for increases in food and animal feed prices resulting from the diversion of food crops, such as corn and soybeans, to fuel production; |
| | ethical issues associated with diverting food crops and fertile acreage to fuel production; and |
| | only a modest reduction in carbon dioxide generation due to the energy inefficiency of producing biofuels from food crops. |
Because of the limitations of first generation biofuels, many companies are now working to make fuels from cellulosic biomass rather than from food-based biomass. Cellulosic biomass is found in virtually all plant material, including sustainable non-food crops such as switch grass and wood chips, and agricultural plant wastes such as corn stover and sugar cane bagasse. Cellulosic biomass is comprised of, among other things, cellulose and hemicellulose, which are long chains of six and five carbon sugars, respectively, that are linked together. To access these sugars, biofuels producers typically utilize heat and chemicals to pretreat these cellulosic materials through a variety of processes that expose the hemicellulose and cellulose. Once exposed, these long chains can be broken down into individual sugar units which can be transformed into fuels.
While fuels produced from cellulosic biomass would represent significant advances over first generation biofuels, there have been several challenges in their development. These challenges include converting cellulose and hemicellulose into sugar, which is a more complicated process than converting corn starch and sugar cane into sugar. In addition, biomass sources vary greatly by plant species and geographic region. One of the challenges of advanced biofuels is developing a technology that can convert the great variety of biomass sources found throughout the world to fermentable sugars. Moreover, the yeast that are currently used to convert corn starch and sugar cane into ethanol typically are not capable of converting the different types of sugars that are produced from cellulosic biomass into ethanol. Solving these challenges will require cellulosic biofuels manufacturers to develop innovative, robust biocatalysts that will have greater product yield and be more cost-effective, and will react quickly and continually under industrial conditions. To date, no companies have successfully done this economically and at commercial scale.
Our Solutions for the Biofuels Market
We believe that our technology platform will enable the development of biocatalysts that can be used to produce commercially viable, cellulose-derived biofuel alternatives to petroleum-based fuels. Since 2006, we have been engaged with Shell in a research and development collaboration under which we are developing biocatalysts for use in producing advanced biofuels. Our advanced biofuels program focuses on two primary elements: (1) developing biocatalysts to convert cellulosic biomass into sugars; and (2) converting these sugars into two advanced biofuels, cellulosic ethanol and biohydrocarbon diesel. For the first element, we have used our technology platform to improve our cellulase and other biocatalysts. For the second element, we have developed a biocatalyst that converts sugars to diesel fuel, and are working on improving ethanol-producing yeast. We are using our technology platform to develop biocatalysts that we believe will:
| | increase the rate at which cellulosic biomass is converted into biofuels; |
| | increase the yield of biofuels produced from cellulosic biomass; |
83
Table of Contents
| | eliminate the need to use food resources for the production of biofuels; |
| | provide producers with more flexibility in designing processes to convert cellulosic biomass to biofuels, thereby reducing the costs associated with building and operating biofuel production facilities; and |
| | enable the production of new types of cellulosic biofuels that could be alternatives to petroleum-based fuels. |
Under our research and development collaboration with Shell, Shell will have the right, but not the obligation, to commercialize any technology that we develop in our biofuels program. If Shell commercializes our biofuels technology, we will collect a royalty for every gallon of fuel that Shell produces using our technology. If Shell chooses to commercialize any biofuels products developed through our collaboration, we believe that the combination of our technology platform with Shells proven product development capabilities and resources could enable a biofuels solution that extends from the conversion of cellulosic biomass into biofuels to delivery and distribution of refined biofuels to consumers at the pump.
Sugar Platform
As part of our biofuels research and development collaboration with Shell, we are using our technology platform to develop a suite of cellulases and other biocatalysts to convert cellulosic biomass to sugar, which we sometimes refer to as our sugar platform. One of the goals of our sugar platform is to improve the performance and operational range of cellulases and other biocatalysts so that they cost-effectively function in industrial conditions. For example, we have developed several of our cellulase biocatalysts that now function at temperature and acidity levels that we believe are close to commercial production targets. The benefit of increasing the operational range of the cellulases is to provide maximum flexibility in the design and function of the facility that is used to produce cellulose-derived sugars, thus decreasing the costs of production and lowering the cost of the end product to make it competitive with petroleum-based fuels.
Another goal of our sugar platform is to increase the rate and extent of conversion of cellulosic biomass to fermentable sugars. The more rapidly and efficiently that biocatalysts convert cellulose and hemicellulose to sugars, the less expensive the biomass conversion process will be to operate. We are developing our biocatalysts to produce more sugar per unit volume. For example, we have developed cellulase biocatalysts that we believe produce twice as much sugar from cellulose as a leading commercially available product. We believe faster sugar production from our biocatalysts will lower capital costs and production costs and result in lower-cost sugar to convert to an end fuel product.
We are developing a library of cellulases that have the potential to convert a wide variety of cellulosic biomass sources into fermentable sugars. The cellulosic biomass that we expect will be used to produce advanced biofuels is highly variable from region to region and can change over time. To optimize the local and seasonal conversion of biomass to fermentable sugars, we expect to use technology similar to our Codex Biocatalyst Panel of cellulases that Shell can use to customize the biocatalysts that they use at each advanced biofuel production facility. This technical innovation may ultimately make our sugar platform feedstock agnostic. In addition, we licensed a commercial-scale enzyme production system from Dyadic in 2008 that we expect will enable the cost-effective production of the high-performing biocatalysts that we are developing for Shell. We believe that the combination of our high-performing cellulases and other biocatalysts, the feedstock flexibility that we expect our Codex Biocatalyst Panels will provide, plus the ability to produce these biocatalysts cost-effectively at commercial scale will enable us to develop a scalable, global sugar platform that will provide a competitive advantage in the advanced biofuels market.
Cellulosic Ethanol
The goal of our cellulosic ethanol program is to develop commercial yeast that rapidly produces high levels of ethanol from cellulose-derived sugars. Cellulosic biomass produces several types of sugars,
84
Table of Contents
including glucose, xylose and arabinose. Xylose is typically found in sugars derived from cellulosic biomass and is not converted by the yeast currently used in todays first generation ethanol production. Therefore, it is important to develop yeast that can convert xylose and other sugars into ethanol. Using a number of our core technologies, including whole genome shuffling and cellular engineering, we are working with a variety of active industrial and laboratory yeast strains to develop a xylose-utilizing yeast strain that converts most of these sugars to ethanol, which should result in greater ethanol production and lower capital and ethanol production costs.
Biohydrocarbon Diesel
We have made significant advancements in our biohydrocarbon diesel fuel program, which is focused on converting cellulose-derived sugar into a fungible diesel blending stock. Based on our testing to date, our biocatalysts rapidly produce high quantities of fuel product per unit volume, which has the potential to reduce production costs and increase the efficiency and productivity of the biohydrocarbon manufacturing process. Our biohydrocarbon program has several additional advantages that could lower the production costs of diesel fuel. Our diesel-producing microbe secretes the diesel molecule from the cell, which then separates from the media in which the cell lives and grows. As a result, our production system can be run continuously without having to stop fuel production to harvest the fuel and purify the fuel product. We believe that many other comparable diesel-producing systems must isolate the fuel-producing cells, break-open the cells to release the fuel and purify the fuel from the resulting mixture, which significantly increase production costs for the end fuel product. In addition, we believe that the biohydrocarbon fuel product that we develop will be able to be blended directly into existing diesel fuel with little or no additional processing at a refinery, which would further lower production costs. In contrast, existing biodiesel fuel that is derived from plant oils must be chemically modified before they are suitable for use as diesel components. These chemical modifications involve processing steps before such fuel is ready for use, which adds to the cost of producing the fuel. In addition, other advanced biofuel programs aimed at producing diesel alternatives require extensive and difficult hydrogenation reactions, which are expensive and require capital intensive facilities that are not widely available.
In contrast to biodiesel produced from plant oils, we expect that the diesel fuel that we develop will be compatible with the existing transportation infrastructure, including distribution systems. A new fuel that works in existing engines and fuel production and distribution systems will not require additional investment in infrastructure to deploy this new technology. As discussed above, we believe that the diesel fuel that we develop will be capable of being blended in conventional petrochemical refineries that are widely used across the globe. This production flexibility should reduce structural barriers to adoption of the molecule as a wide-spread petroleum alternative.
Additional Bioindustrial Opportunities
We believe that our technology platform, together with the knowledge and experience gained from our efforts in the pharmaceutical market and in our biofuels development program, will allow us to capitalize on opportunities in other bioindustrial markets, including carbon management, water treatment and chemicals. Depending on the market, we may pursue collaborations with industry leaders to allow us to leverage their competitive strengths and resources in pursuit of these opportunities.
Carbon Management
During the 20th century, global surface temperature increased 0.74 ± 0.18 degrees Celsius. In 2007, the Intergovernmental Panel on Climate Change concluded that most of this temperature increase was due to increasing concentrations of greenhouse gases, including carbon dioxide, which resulted from human activity. The consensus of the world scientific community is that continued climate change during this century will harm the global environment in unpredictable and potentially catastrophic ways. While a number of critics contest these conclusions, the global pressure to reduce carbon dioxide emissions is
85
Table of Contents
dramatic and increasing. Emissions continue to rise, even as the global demand for regulation grows. According to the EIA, the global emission level of carbon dioxide is projected to rise from 29 billion metric tons in 2006 to 33 billion metric tons in 2015 and 40 billion metric tons in 2030. Of the approximately seven billion tons of carbon dioxide equivalents emitted by the United States each year, approximately 40% is produced by the electric power industry. Furthermore, the share of global carbon dioxide emissions by the electric power industry could potentially increase in the future as growing demand for power increases alongside a growing population. By 2030, the EIA estimates, China and India will account for 34% of the worlds carbon dioxide emissions, driven largely by their use of coal in generating electricity. The need for a viable method to manage these growing carbon dioxide emissions represents a significant opportunity.
In the carbon management market, we are seeking to apply our technology platform to the management of carbon dioxide emissions from stationary point sources such as coal-fired power plants. As part of this effort, in December 2009, we entered into an exclusive joint development agreement with CO2 Solution Inc. under which we will combine our biocatalyst-enabled technology platform with CO2 Solutions proprietary enzymatic methods for the efficient capture of carbon dioxide from coal-fired power plants and other large sources of carbon dioxide emissions. We believe our biocatalysts have the potential to enhance the effectiveness of CO2 Solutions carbon capture processes in harsh industrial conditions.
To further our efforts in the carbon management market, we have filed provisional patent applications relating to biocatalysts that we believe may optimize the process of removing carbon dioxide from flue gases. These biocatalysts improve the effectiveness of amine solvents, one of the leading potential technologies to remove carbon dioxide from flue gas. A major drawback of amine solvent technologies is the additional parasitic energy required to operate them. Based on initial models, we believe that our biocatalysts may reduce this parasitic energy loss by up to 35%. In the laboratory, these biocatalysts have also exhibited increased tolerance for flue stack-type operating conditions, though not yet at target commercial levels. Although our research is in its early stages, we believe that it may be possible to cost-effectively utilize biocatalyst-enabled solutions to separate carbon dioxide from other exhaust gases and direct them to separate sequestration mechanisms.
Water Treatment
Water treatment is another example of a potential major market opportunity for novel biocatalyst-enabled solutions. According to a United Nations study published in March 2007, approximately 80% of all diseases in the developing world are caused by unsafe water and poor sanitation. In addition, industrial manufacturing operations and municipal water usage generate large quantities of waste water, which must be treated in order to avoid contamination of our fresh water resources and our oceans. There are many sources and types of water pollution, and when different types of pollution mix together it presents complex and challenging remediation problems downstream.
The market for biocatalysts in water treatment is in a very early stage of development. However, new interest in biocatalyst-enabled solutions in water treatment has been sparked in part by concerns about possible contamination of drinking water from industrial and other sources. For example, a U.S. government report released in 2006 examined the potential of biocatalysts in the treatment of groundwater and drinking water in both civilian and military applications. The report concluded that biocatalyst- embedded water filters held significant promise for the treatment of agents, pesticides, or other chemical contaminants in drinking water systems, as well as for the decontamination of pipes and other equipment with contaminant residue. We believe that there are also opportunities for biocatalyst-enabled solutions to treat municipal wastewater streams.
86
Table of Contents
Chemicals
There are also significant market opportunities in the chemical industry for companies that can help reduce or eliminate petroleum dependency, as well as costly and wasteful manufacturing processes. For example, according to the EIA, in 2008, approximately 214 million barrels of petroleum were used in petrochemical feedstocks.
We believe that fermentable sugars produced from cellulosic biomass may serve as an alternate source of carbon for use in the manufacture of many chemicals. This potential market may provide an opportunity to leverage our funded work with Shell into a separate business in the non-fuels chemicals industry. Our license agreement with Shell permits us to use technology developed for Shell outside of the field of fuels and lubricants. In addition, our technology platform could be applied to develop biocatalysts for the conversion of sugar or other feedstocks, rather than petroleum-derived hydrocarbons, into commercially important chemicals. We have rights to pursue a number of chemical market opportunities under our license agreement with Maxygen. To pursue certain other opportunities in the chemicals market, we will need to license additional rights from Maxygen.
Strategic Collaborations
Our strategic collaborations allow us to expand into new markets and to service our existing customers, while operating our business with maximum capital efficiency. By collaborating with companies such as Arch and Shell, we are able to leverage both our technology platform and our collaborators strengths in production and distribution. This allows us to focus our capital on key areas such as research and development.
Arch
We are collaborating with Arch Pharmalabs Limited, or Arch, of Mumbai, India in the manufacture and sale of intermediates and APIs produced using our biocatalyst-enabled processes. Arch has extensive expertise in chemical process development and scale-up, and is a leading producer of intermediates and generic APIs in India.
We were previously party to agreements with Arch pursuant to which Arch manufactured and supplied ATS-8 for us and on our behalf and which we paid Arch a percentage of the profits we earned on our sales of ATS-8. In August 2008, with the exception of the Master Services Agreement with Arch entered into as of August 1, 2006, we simultaneously terminated all of our existing agreements with Arch and entered into a series of new agreements with Arch, significantly expanding the relationship between the parties. These new agreements include a biocatalyst license and development agreement, a biocatalyst supply agreement, product supply and marketing agreements, and various ancillary agreements, which we refer to as the Arch Agreements. Under the terms of the Arch Agreements, we are developing and supplying certain biocatalysts to Arch for use in the manufacture of atorvastatin, azetidinones, montelukast, duloxetine and phenylephrine, and intermediates in their manufacture, which we refer to as the Collaboration Products. We granted Arch the exclusive right to use these biocatalysts to manufacture the Collaboration Products, except for duloxetine and intermediates used in its manufacture, for which we granted Arch a non-exclusive right. Arch agreed to manufacture and supply the Collaboration Products exclusively for and on behalf of us, except that Arch retained the right to supply atorvastatin to one customer under a prior agreement between Arch and that customer. We agreed to obtain all Collaboration Products, other than duloxetine and intermediates used in its manufacture, exclusively from Arch. We have sales and marketing arrangements with Arch in various countries.
Each party can terminate the Arch Agreements after ten years by providing the other party with three years prior written notice beginning at year seven of the Arch Agreements. Each party also has the right to terminate the Arch Agreements or convert the exclusive rights in the Arch Agreements to non-exclusive rights in their entirety or on a product-by-product basis in the case of certain material breaches by the other
87
Table of Contents
party either immediately or if such breach is uncured within 45 days, depending on the nature of the breach. Additionally, if certain economic and tax assumptions render performance under the Arch Agreements economically unviable, each party can terminate the Arch Agreements upon three years notice at any time during the term of the Arch Agreements.
We plan to enter into additional agreements with Arch to manufacture additional intermediates and APIs, including the manufacture of products for innovator customers.
Shell and Other Biofuels Partners
We collaborate with Equilon Enterprises LLC dba Shell Oil Products US, or Shell, to develop commercially viable fuels from cellulosic biomass. If Shell chooses to commercialize any biofuels products developed through our collaboration, we believe that the combination of our technology platform with Shells proven project development capabilities and resources could enable a biofuels solution, from converting cellulosic biomass into biofuels that extends to delivering and distributing refined biofuels to consumers at the pump.
Shell purchased approximately $3.0 million of our Series D preferred stock in November 2006, approximately $30.5 million of our Series E preferred stock in November 2007 and approximately $30.0 million of our Series F preferred stock in March 2009. In addition, in November 2007, Shell exercised a warrant issued in November 2006 to purchase 428,571 shares of our Series D preferred stock for $3.0 million.
In November 2006, we entered into a research agreement and a license agreement with Shell. After exceeding targets related to biocatalyst performance under the research agreement, we entered into a new research and development collaboration under a five year amended and restated collaborative research agreement in November 2007, which was amended further in March 2009. Under the terms of the amended and restated collaborative research agreement, we agreed to use our proprietary technology platform to discover and develop biocatalysts for use in converting cellulosic biomass into biofuels and related products. We received an up-front payment of $20 million in 2007 upon signing the amended and restated collaborative research agreement. We have agreed to work exclusively with Shell until November 2012 to convert cellulosic biomass into fermentable sugars that are used in the production of fuels and related products and to convert these sugars into fuels and related products. However, Shell is not required to work exclusively with us, and could develop or pursue alternative technologies that it decides to use for commercialization purposes instead of any technology developed under our collaborative research agreement with Shell. Even if Shell decides to commercialize products based on our technologies, they have no obligation to purchase their biocatalyst supply from us. The up-front fee is refundable under certain conditions, such as a change in control in which we are acquired by a competitor of Shell. This refundability lapses ratably on a straight-line basis over a five-year period which started in November 2007 and which ends in November 2012.
In 2009, we agreed to devote to the research and development collaboration FTEs by March 2009, which are required to be funded by Shell at an annual base rate per FTE of $ for FTEs located in the United States, and $ for FTEs located in Hungary. These annual base rates per FTE are subject to annual increases of each subsequent year of the collaboration. Until November 1, 2010, Shell has the right to reduce the number of funded FTEs under the collaborative research agreement by up to FTEs following 60 days advance written notice. After November 1, 2010, Shell has the right to further reduce the number of funded FTEs, with any one reduction not to exceed funded FTEs, following advance written notice. The required notice period ranges from 30 to 270 days, so the earliest an FTE reduction could take place would be December 2, 2010. Following any such reduction, Shell is subject to a standstill period of between 90 and 360 days during which period Shell cannot provide notice of any further FTE reductions. The notice and standstill periods are dependent on the number of funded FTEs reduced, with the length of notice and standstill periods increasing commensurate with the number of FTEs reduced. To date, Shell has not reduced the number of funded FTEs. We are also eligible for milestone
88
Table of Contents
payments of up to $ over the remaining term of the agreement, contingent upon the achievement of certain technical goals beginning in 2009, and a milestone payment of $ upon achievement of certain commercial goals. Our technical goals have included filing patent applications relating to our development program, and matching predetermined benchmarks for the production of sugars from pre-treated cellulosic biomass using our cellulases and the production of a biohydrocarbon diesel component for sugar derived from cellulosic biomass. We have met or exceeded each of our milestones to date. We believe that several of our cellulase biocatalysts now function at temperatures and acidity levels that are close to the commercial targets. We also believe that our cellulase biocatalysts produce twice as much sugar from pre-treated cellulosic biomass as leading commercially available products under target industrial conditions.
Shell can terminate the amended and restated collaborative research agreement after November 1, 2010, for any or no reason by providing us with at least nine months notice. We will have the right to terminate the amended and restated collaborative research agreement upon 90 days notice if Shell decides to fund less than a certain number of our FTEs in the performance of activities under the amended and restated collaborative research agreement and provided certain other conditions are met. Each party also has the right to terminate the amended and restated collaborative research agreement in the case of a breach by the other party if such breach is uncured within 60 days. Each party also can terminate the amended and restated collaborative research agreement if such party believes the other party has assigned the amended and restated collaborative research agreement to a direct competitor of such party in the field of converting cellulosic biomass into fermentable sugars that can be converted into fuels and related products.
Under our agreements with Shell, we retain ownership of all intellectual property we develop, other than patent rights related to certain fuel innovations, and Shell will have an exclusive license to such intellectual property we develop. If we acquire or license technology from third parties for the purpose of these research activities, we will own or control such intellectual property while Shell will be granted a license in its field of use for research and commercial use consistent with the licenses granted to Shell, under the license agreements.
In November 2006, we also entered into a license agreement with Shell, which was amended and restated in November 2007, and further amended in March 2009. Under the terms of the amended and restated license agreement, we granted to Shell, a worldwide, exclusive, royalty-bearing license, including the right to grant sublicenses, to manufacture, have manufactured, use, sell, offer for sale and import any product covered by our patents or which utilizes our technology for use in the field of converting cellulosic biomass into biofuels and related products. The patents and technology licensed include our then existing patent rights and technology and patent rights and technology developed or acquired during performance of the research agreement, in each case related to converting cellulosic biomass into biofuels and related products. We additionally granted Shell royalty-free licenses which allow Shell to manufacture or have manufactured biocatalysts developed under the research agreement solely for the purposes of using such biocatalysts in the manufacture of products for use in the field of converting cellulosic biomass into biofuels and related products, such licenses to be used only in accordance with the royalty-bearing license described above. These royalty-free licenses are (i) an exclusive license under the patents and technology related to converting cellulosic biomass into biofuels and related products and developed or acquired by during performance of the research agreement and (ii) a non-exclusive license to patents and technology controlled by us that are necessary or useful for converting cellulosic biomass into biofuels and related products.
Shell will be required to pay us a royalty per gallon with respect to certain fuel products manufactured using our technology platform, including liquid fuels, fuel additives and lubricants, if Shell or any of its licensees manufactures such products. The applicable fuel products are those products which are covered by patents or utilize technology related to converting cellulosic biomass into biofuels and related products that were either developed or acquired during performance of the research agreement or are controlled by us and necessary or useful for such purpose. With respect to cellulosic biomass converted into sugars, Shell agreed to pay us a royalty per gallon of fuel product made from those sugars. With respect to sugars
89
Table of Contents
converted into fuel, Shell agreed to pay us a separate royalty per gallon of fuel product made from those sugars. We may be entitled to receive one or both of these royalties depending on whether Shell uses our technology to commercialize one or both of these steps.
Shell can terminate the amended and restated license agreement for any or no reason by providing us with six months notice. If Shell terminates the license agreement, Shell will no longer have the right to use any of our biofuels technology. Each party also has the right to terminate the amended and restated license agreement in the case of a breach by the other party if such breach is uncured within 60 days. The duration of the license agreement differs for each of the fields of use covered by the license agreement, but for each field of use it continues until the later of (i) 20 years after the first sale of product licensed under the agreement in the field of use or (ii) expiration of the last to expire patents covering products licensed under the agreement in the field of use that were either developed or acquired during performance of the research agreement or are controlled by us and necessary or useful for such purpose.
One element of our collaboration with Shell relates to the development of cellulosic ethanol. In connection with our collaboration with Shell, we entered into a collaborative research and license agreement with Iogen Energy Corporation, or Iogen, and Shell in July 2009. Under the collaborative research and license agreement with Iogen and Shell, we agreed to collaborate with Iogen and Shell to develop technology relating to the conversion of cellulosic biomass to ethanol and to implement this technology at commercial scale. We and Iogen will jointly own any inventions arising under the research activities pursuant to the collaborative research and license agreement, except that inventions relating to one partys core technology will be solely owned by that party and licensed to the other party. Inventions that we own under the collaborative research and license agreement are subject to the licenses granted by us to Shell, as well as the payments from Shell to us, under our other agreements with Shell. Iogen has agreed to pay us a royalty per gallon with respect to certain fuel products, which include liquid fuels, fuel additives and lubricants, that are covered by inventions jointly made by us and Iogen, but that are solely owned by Iogen. We will be entitled to collect royalties from Shell for any use of our biofuels technology by Shell or Iogen. Shell can choose to commercialize cellulosic ethanol manufactured using our technology independently, or in collaboration with Iogen.
The term of the collaborative research and license agreement with Iogen and Shell shall continue until expiration or termination of our license agreement with Shell or of Iogens technology license agreement with Shell. Shell can terminate the collaborative research and license agreement for any or no reason by providing us and Iogen with 30 days notice. Each party also has the right to terminate the collaborative research and license agreement in the case of breach by another party if that breach is uncured within 60 days.
We have acquired access to a fungal expression system that is capable of producing biocatalysts at commercial scale through a license agreement with Dyadic International, Inc. and its affiliate, or Dyadic, in November 2008. Under the license agreement with Dyadic, we obtained a non-exclusive license relating to Dyadics proprietary fungal expression technology for the production of biocatalysts. We also obtained access to specified materials of Dyadic relating to this Dyadic technology. Our license is sublicenseable to Shell in the field of biofuels. Each party agreed that neither it nor its affiliates or sublicensees will assert any claim of infringement of any patent covering improvements to the Dyadic materials that were made by that party or its affiliates or sublicensees against the other party, or its affiliates, sublicensees, successors, distributors, or customers. We agreed to pay Dyadic certain license issuance fees, milestone payments, and fees based on volume of product manufactured using this Dyadic technology. We have the right to terminate the license agreement at will upon notice after payment of the license issuance fees. Either party has the right to terminate the license agreement for a material breach of the other party that is uncured within a period of time after notice. Dyadic has the right to terminate our licenses under the license agreement if we challenge the validity of any of the patents licensed under the license agreement. Our licenses, and access to Dyadics materials, under the license agreement will terminate as a result of any termination of the license agreement other than due to Dyadics material breach.
90
Table of Contents
Technology
We are innovators in the directed evolution of enzymes and microbes to enable industrial biocatalytic reactions and fermentations via biocatalyst engineering, metabolic pathway engineering and fermentation microbe improvement. Our technology platform has enabled commercially viable products and processes for the manufacture of pharmaceutical intermediates, and we are in the process of applying our technology platform in connection with the development of biofuels.
Our approach to developing commercially viable biocatalytic processes begins by conceptually designing the most economically practical manufacturing process for a targeted product. We then develop optimized biocatalysts to enable that process design, using our directed evolution technology, including screening and validating biocatalysts under relevant conditions. Typical design criteria include stability in the desired reaction conditions, biocatalyst activity and productivity (yield), ease of product isolation, product purity and cost. Alternative approaches to biocatalytic process development typically involve designing and engineering the biocatalytic processes around shortcomings of available biocatalysts, including, for example, biocatalyst immobilization (for stability and/or reuse), special equipment and costly product isolation and purification methods. We circumvent the need for these types of costly process design features by optimizing the biocatalyst for fitness in the desired process environment. As a result, we enable and develop cost-efficient processes that typically are relatively simple to run in conventional manufacturing equipment. This also allows for the efficient technical transfer of our process to our manufacturing partners.
The successful embodiment of our technology platform in commercial manufacturing processes requires well-integrated expertise in a number of technical disciplines. In addition to those directly involved in practicing our directed evolution technologies, such as molecular biology, enzymology, microbiology, cellular engineering, metabolic engineering, bioinformatics, biochemistry, and high throughput analytical chemistry, our process development projects also involve integrated expertise in organic chemistry, chemical process development, chemical engineering, fermentation process development, and fermentation engineering. Our tightly integrated, multi-disciplinary approach to biocatalyst and process development is a critical success factor for our company.
Enzyme Optimization Overview
The enzyme optimization process starts by identifying genes that code for enzymes known to have the general type of catalytic reactivity for a desired chemical reaction. Typically, we identify gene sequences in published databases and then synthesize candidate genes having those sequences. Using a variety of biotechnology tools, we diversify these genes by introducing mutations, giving rise to changes in the enzymes for which they encode. The methods for diversifying these genes, and types of diversity being tested, often vary over the course of a biocatalyst optimization program. For finding initial diversity, methods typically include random mutagenesis and site-directed (included structure-guided) mutagenesis. We also test mutational variations that distinguish related enzymes among different organisms. Once we have identified potentially beneficial mutations, we test combinations of these mutations in libraries made using our proprietary gene recombination methodologies, gene shuffling and multiplexed gene SOEing.
With our proprietary gene shuffling methodology, we generate libraries of genes that have random combinations of the mutations we are testing. The pool of genes is used to transform host cells, which entails introducing the various genes, one each, into host cells. These cells are then segregated and grown into colonies. Cells from individual colonies are cultured in high throughput to produce the enzyme encoded by the shuffled gene in those cells. The enzymes are then screened in high throughput using test conditions relevant to the desired process. The screening results identify individual shuffled genes that produce improved enzymes having combinations of beneficial mutations and weed out enzymes having detrimental ones. Using different test conditions and/or different analytical methods, we can identify
91
Table of Contents
variant enzymes that exhibit various improved performance characteristics, such as stability, activity and selectivity, under conditions relevant to the desired chemical process.
In the next step in our optimization process, we use our proprietary software tool, ProSAR, to analyze protein sequence-activity relationships. We initially licensed ProSAR from Maxygen and further developed and customized ProSAR to address our specific needs. ProSAR aids in identifying specific gene and enzyme mutations that are beneficial, neutral or detrimental with respect to the desired performance characteristics. Earlier directed evolution methods did not separately evaluate individual mutations in libraries of variants which carry multiple mutations, where beneficial and detrimental performance characteristics may be mixed in an individual gene or enzyme. Capitalizing on the advent of inexpensive gene sequencing, we are able to determine which particular mutations are present in the genes and proteins we have screened. Our ProSAR bioinformatics software relates the screening results to the mutations and ranks the individual mutations with regard to their degree of benefit or detriment, relative to whichever process parameter(s) the screening tested. Using that information, we can bias the pool of mutational diversity in the next iteration to further the accumulation of beneficial diversity and cancel out detrimental diversity in the individual genes in the resulting shuffled library. The ProSAR results also help us develop ideas about new diversity to test. ProSAR, combined with efficient gene synthesis and high quality library generation methods, has led to a significant increase in the efficiency and speed of enzyme improvement and optimization.
In another step of our optimization process, we take the best variants we have identified and prepare enough of each to test in the desired chemical process at laboratory scale, for in-process confirmation. This optimization routine is done iteratively, typically adding new diversity to the pool in each iteration. The gene that codes for the best performing enzyme in one iteration is used as the starting gene for the next iteration of shuffling and screening. As the enzymes improve over these iterations, the screening conditions are made increasingly more stringent. In this way, enzyme biocatalysts are rapidly optimized until all in-process performance requirements have been achieved and the economic objectives for the desired process have been met.
Mutiplexed gene SOEing is our new proprietary methodology for rapidly generating gene variants. Using multiplexed gene SOEing, we rapidly generate collections of individual gene variants that have predetermined, as opposed to random, combinations of mutations we are testing. It is based on a biotechnology technique, which we refer to as SOEing, or Splicing by Overlap Extension, generally used to make a hybrid, or spliced, gene from fragments of two genes and/or to introduce a specific mutation into a splice between fragments of one gene. We have automated the process to robotically make, in parallel, one hundred to several hundred variants, each with a predetermined combination of the mutations we are testing. The variants are introduced into host cells, and the encoded enzyme is produced and screened in high throughput, as described above.
Using multiplexed gene SOEing, we can test many mutations and combinations thereof in parallel, and because the mutation incorporation is controlled and predetermined before screening, as opposed to random incorporation and selection after screening, the resulting data set can be more optimal for ProSAR analysis.
We believe using multiplexed gene SOEing to quickly survey many mutations, followed by ProSAR-driven shuffling of beneficial mutations, is a particularly effective approach, providing rapid gains in enzyme performance.
Codex Biocatalyst Panels
Our Codex Biocatalyst Panels were initially developed to speed our own internal process for identifying enzymes with desired characteristics for further optimization. Each Codex Biocatalyst Panel is comprised of variants of one or more enzymes that catalyze one type of a generally useful chemical
92
Table of Contents
reaction. We assemble, on one or more microtiter sample plates, variants of a parent enzyme that we pre-optimize for stability in industrial chemical processes and for ready manufacturability. The variants are diversified to react to a variety of chemical structures that are susceptible to that type of chemical reaction.
Either we or our innovator pharmaceutical customers use the Codex Biocatalyst Panels to screen a new chemical structure against the assembled variants to rapidly identify variants that react with the new chemical structure. For some new structures, a variant on the panel could enable production of the desired product. We can also analyze the data from the panel screen using ProSAR to identify the mutations that are beneficial for the reaction of the new structure and further optimize the enzyme as needed using the enzyme optimization techniques described above. In cases where a customer wishes to screen a proprietary new chemical structure itself, we can produce a custom panel of new variants on a sample plate produced by multiplexed gene SOEing.
We may also use our Codex Biocatalyst Panels in our bioindustrial programs. In our biofuels research and development collaboration with Shell, we are developing a library of cellulases that have the potential to convert a wide variety of cellulosic biomass sources into fermentable sugars. The cellulosic biomass that we expect will be used to produce advanced biofuels is highly variable from region to region and can change over time. To optimize the local and seasonal conversion of cellulosic biomass to fermentable sugars, we expect to produce a Codex Biocatalyst Panel of cellulases that we or Shell can use to customize the biocatalysts that Shell uses at each advanced biofuel production facility. This technical innovation may ultimately make our sugar platform feedstock agnostic. Similarly, there is regional variation in coal. We may develop a Codex Biocatalyst Panel that we or our customers can use to tailor our carbon capture biocatalysts to the specific characteristics of the coal used in each energy facility that adopts our carbon capture technology.
Microbe Optimization using Gene Optimization
For fermentation microbes, we enhance metabolic pathways by using gene optimization to improve the production and/or productivity of one or more enzymes in a series of in vivo reactions that make a desired product. We optimize the gene/enzyme as described above using either in vitro or in vivo screening. For fermentation applications, the microbes containing the improved gene(s) are directly evaluated in laboratory scale fermenters.
The metabolic pathway may naturally exist in the microbe, but productivity and/or selectivity improvements are needed to economically produce more of the desired natural product and/or less of an undesired by-product. We can also introduce a new metabolic pathway to produce a desired product using our gene shuffling technology in combination with synthetic biology, a type of metabolic engineering in which new genes are introduced into a microbe.
We are using our gene/enzyme optimization methodologies in our biofuels program to optimize fermentation microbes, including optimization of:
| | native and introduced (non-native) cellulase genes for increased productivity in our cellulase production microbes; |
| | an introduced (non-native) pathway in yeast for the conversion of xylose, a cellulose-derived sugar, to ethanol; and |
| | an introduced (non-native) pathway in a microbe for the production of our biohydrocarbon fuel molecule. |
Microbe Optimization using Whole Genome Shuffling
In addition to our gene optimization technology for enzymes, we have another complimentary technology in our platform for the optimization of fermentation microbes called Whole Genome Shuffling. Whole Genome Shuffling allows us to improve the performance of a fermentation microbe by shuffling
93
Table of Contents
unidentified mutations in unidentified genes across the genome. We start with a diversity of mutational variants of a fermentation organism, generated by conventional means such as random mutagenesis. Our Whole Genome Shuffling involves introducing the entire genome of two or more such cells into a single cell, in which the genetic machinery of the combined cell recombines, or shuffles, the genomes. In one method, this is accomplished by protoplast fusion, in which the cell walls are removed to leave the cells contents contained only by their cell membranes. The cell membranes of these protoplasts in the diverse population are induced to fuse together into fusants containing the genome of two or more of the parent cells. From these fusants, we regenerate normal cells, each with one copy of a hybridized genome. Microbial colonies are then grown and screened for their performance in the fermentative production of the desired product. This process can be repeated, including with the introduction of new mutations, until the desired performance in the fermentation process is achieved. One of our collaborators is operating a fermentation process for a generic pharmaceutical product using microbes we developed by Whole Genome Shuffling.
We are using our Whole Genome Shuffling technology in our biofuels program to optimize fermentation microbes, including optimization of:
| | enzyme production hosts for increased production of cellulase enzymes; |
| | ethanol-producing yeasts for improved xylose utilization, ethanol productivity, and tolerance to higher ethanol concentrations; and |
| | our biohydrocarbon producing strain for increased productivity. |
Metabolic Engineering and Synthetic Biology
In addition to our proprietary enzyme and microbe optimization technologies, we have built expert capabilities in a suite of new metabolic engineering technologies for the development and optimization of fermentation microbes. These technologies are generally applicable to our pathway and strain engineering programs. Genomics, transcriptomics, proteomics and metabolomics all provide more in-depth analyses of the metabolic functioning of fermentation microbes, and differences between variants, to guide further improvements. In many cases, these analyses help to identify enzymes that need to be modified (removed, increased, stabilized, or otherwise modified) in order to increase the overall productivity and performance of the strain.
Synthetic biology involves the design, synthesis and introduction of new genetic programming to organisms for new biological functions. This field has rapidly developed in recent years as DNA synthesis and sequencing costs have rapidly dropped. Using synthetic biology, we are taking advantage of the exploding publicly available gene and genome sequence information in our gene and metabolic pathway optimization projects. This information is being leveraged by our ProSAR software and multiplexed gene SOEing methodologies. For example, we use synthetic biology in our biofuels program to introduce non-native pathways for xylose utilization and for biohydrocarbon production and to optimize these pathways.
License Agreement with Maxygen
In March 2002, we licensed from Maxygen core enabling technology. The license agreement was amended in September 2002, October 2002 and August 2006.
Under the terms of this license agreement, Maxygen granted us a worldwide, exclusive, license, with a right to sublicense, under certain Maxygen intellectual property related to the use of shuffling technology in a variety of fields of use. This license includes the right to develop, make, have made, use, import, have imported, offer for sale, sell, otherwise commercialize or distribute biocatalysts for the manufacture of generic and branded pharmaceuticals, certain classes of chemicals and certain applications related to energy and biofuels. Under the license agreement, Maxygen also provided us with certain biological
94
Table of Contents
materials to facilitate use of the gene shuffling technology. We can use the licensed Maxygen shuffling technology in a wide variety of organisms including algae, bacteria, cyanobacteria, fungi and yeasts, but we are restricted from using the technology in land plants. Our license is exclusive with respect to bacteria, yeast and fungi, but is nonexclusive with respect to algae and cyanobacteria. The Maxygen license extends for the lifetime of the patents included in the Maxygen intellectual property plus an additional 50 years for any know-how or materials included in the license agreement, unless earlier terminated.
The license agreement also specifically excludes us from certain activities. Under the terms of this license agreement, our license is subject to certain third-party rights in the Maxygen shuffling technology and we cannot utilize the licensed Maxygen shuffling technology for drug discovery or for the manufacture of protein-based therapeutics, such as antibodies.
Under the terms of our license agreement with Maxygen, we are obligated to pay Maxygen a significant portion of certain types of consideration we receive in connection with our biofuels research and development, including our collaboration with Shell. The actual fees payable to Maxygen will depend on the amount, timing and type of consideration we receive, including payments from the sale of our equity securities to Shell and payments in connection with the sale of fuel products made with a biocatalyst developed using the licensed technology and/or research and development activities.
If we directly commercialize an energy product that is made using any biocatalyst developed from the technology licensed from Maxygen, we will owe Maxygen a 2% royalty on our net sales of the energy product and on amounts received from any sublicense or third party for the use of the energy product, to the extent that we utilize such energy product to provide services to such sublicense or third party. If we sublicense our rights under the license agreement to a third party for the development and commercialization of an energy product, we will owe Maxygen 20% of all consideration we receive from any sublicensee. Specifically, we will owe Maxygen fees in connection with consideration we receive in the form of (1) up-front option and/or license fees, (2) FTE funding for biofuels research, (3) milestone payments, (4) payments from the sale of our equity securities and (5) payments in connection with the commercialization of energy products made with a biocatalyst developed using the licensed technology.
In the case of consideration received from the sale of our equity securities to Shell, we are obligated to pay Maxygen 20% of any excess paid above $3.97 per share, the price per share of our Series D preferred stock. With regard to FTE funding, we are only obligated to pay Maxygen to the extent the consideration received exceeds a specified amount, which was initially $ per year starting in November 2006, but is adjusted annually . We are also obligated to reimburse up to 20% of the costs incurred by Maxygen related to the prosecution and maintenance of the patents licensed from Maxygen relating to our core technology. Further, in the event that any subsidiary or affiliate of ours develops and/or sells any energy applications using the Maxygen technology, we are obligated to transfer to Maxygen a percentage of the value of the subsidiary or affiliate that is attributable to the Maxygen technology and give Maxygen an option to acquire a percentage of the other consideration that we invest in such affiliate or subsidiary.
In connection with all consideration received from Shell relating to our biofuels research and development collaboration, we were obligated to pay Maxygen $7.8 million and $1.0 million for 2007 and 2008, respectively, of which $ and $ , respectively, were payments owed to Maxygen in connection with Shells FTE funding. For the nine months ended September 30, 2008 and 2009, we were obligated to pay Maxygen $0.6 million and $4.2 million, respectively, of which $ and $ , respectively, were payments owed to Maxygen in connection with Shells FTE funding. The payments relating to FTE funding were less than 5% of the total FTE payments we received from Shell in those periods.
Maxygen granted Novo Nordisk A/S certain rights under its intellectual property on September 17, 1997. This grant was later amended and these rights were later assigned by Novo Nordisk to Novozymes A/S and by Maxygen to us. Under this license, Maxygen granted exclusive rights to Novozymes that are outside the field of use licensed to us by Maxygen. Maxygen also granted certain rights to Novozymes co-exclusively in other fields that could overlap with certain fields we are pursuing under our license,
95
Table of Contents
including biofuels. At a minimum, we enjoy co-exclusive rights in such fields and have sufficient rights for our collaborations and partnerships. Novozymes did not receive a license to all of the rights we are using in biofuels applications and which we believe are critical to pursuing such applications.
In exchange for this license, we issued a total of 999,000 shares of common stock and six million shares of Series A preferred stock to Maxygen. As of September 30, 2009, Maxygen beneficially owned approximately 21.6% of our common stock.
Intellectual Property
Our success depends in large part on our proprietary products and technology under which we seek protection from patent, copyright, trademark and trade secret laws. Such protection is also maintained using confidential disclosure agreements. Protection of our technologies is important for us to offer our customers and partners proprietary services and products unavailable from our competitors, and to exclude our competitors from practicing technology that we have developed or exclusively licensed from other parties. For example, our ability to supply innovator pharmaceutical manufacturers depends on our ability to supply proprietary enzymes or methods for making pharmaceutical intermediates or APIs that are not available from our competitors. Likewise, in the generic pharmaceutical area, proprietary protection, through patent, trade secret or other protection of our biocatalysts and methods of producing a pharmaceutical product is important for us and our customers to maintain a lower cost production advantage over competitors. If competitors in our industry have access to the same technology, our competitive position may be adversely affected. As of September 30, 2009, we owned or had licensed rights to approximately 235 issued patents and approximately 280 pending patent applications in the United States and in various foreign jurisdictions. The earliest that any of our intellectual property rights will expire is 2014. Of the licensed patents and patent applications, most are owned by Maxygen and exclusively licensed to us for use in certain fields. These licensed patents and patent applications cover both enabling technologies, as well as patents covering products or methods of producing products. Our licenses to such patents allow us to freely practice the licensed inventions, subject only to the terms of these licenses. The issued patents covering the fundamental shuffling technologies have terms ending as late as 2019. As of September 30, 2009, we owned approximately 35 issued patents and approximately 110 pending patent applications in the United States and in various foreign jurisdictions. These patents and patent applications are directed to our enabling technologies and specific methods and products which support our business in the pharmaceutical and bioindustrial markets. In particular, some of our patents and patent applications are directed to intermediates and processes for the production of pharmaceuticals such as atorvastatin, montelukast and azetidinone compounds. Our U.S. issued patents directed to our enabling technologies have terms that expire from year 2021 to 2024. We continue to file new patent applications, for which terms generally extend 20 years from the filing date in the United States.
We will continue to file and prosecute patent applications and maintain trade secrets as is consistent with our business plan in an ongoing effort to protect our intellectual property. It is possible that our current patents, or patents which we may later acquire, may be successfully challenged or invalidated in whole or in part. It is also possible that we may not obtain issued patents from our pending patent applications or other inventions we seek to protect. We sometimes permit certain intellectual property to lapse or go abandoned under appropriate circumstances. Due to uncertainties inherent in prosecuting patent applications, sometimes patent applications are rejected and we subsequently abandon them. It is also possible that we may develop proprietary products or technologies in the future that are not patentable or that the patents of others will limit or altogether preclude our ability to do business. In addition, any patent issued to us may provide us with little or no competitive advantage, in which case we may abandon such patent or license it to another entity.
Our registered and pending U.S. trademarks include Codexis, Codex and Codex Biocatalyst Panel. The Codexis and Codexis design marks have been registered or are pending in selected foreign countries.
96
Table of Contents
Our means of protecting our proprietary rights may not be adequate and our competitors may independently develop technology or products that are similar to ours or that compete with ours. Patent, trademark, and trade secret laws afford only limited protection for our technology platform and products. The laws of many countries do not protect our proprietary rights to as great an extent as do the laws of the United States. Despite our efforts to protect our proprietary rights, unauthorized parties have in the past attempted, and may in the future attempt, to operate under aspects of our intellectual property or products or to obtain and use information that we regard as proprietary. Third parties may also design around our proprietary rights, which may render our protected technology and products less valuable, if the design around is favorably received in the marketplace. In addition, if any of our products or technology is covered by third-party patents or other intellectual property rights, we could be subject to various legal actions. We cannot assure you that our technology platform and products do not infringe patents held by others or that they will not in the future.
Litigation may be necessary to enforce our intellectual property rights, to protect our trade secrets, to determine the validity and scope of the proprietary rights of others, or to defend against claims of infringement, invalidity, misappropriation, or other claims. Any such litigation could result in substantial costs and diversion of our resources. Moreover, any settlement of or adverse judgment resulting from such litigation could require us to obtain a license to continue to make, use or sell the products or technology that is the subject of the claim, or otherwise restrict or prohibit our use of the technology.
Competition
Overview
We are a leader in the field of directed molecular evolution of biocatalysts. We are aware that other companies, including Verenium Corporation (formed by the merger of Diversa Corporation and Celunol Corporation), Royal DSM N.V., or DSM, Danisco/Genencor, Novozymes, and E.I. DuPont De Nemours and Company, or DuPont, have alternative methods for obtaining and generating genetic diversity or use mutagenesis techniques to produce genetic diversity. Academic institutions such as the California Institute of Technology, the Max Planck Institute and the Center for Fundamental and Applied Molecular Evolution (FAME), a jointly sponsored initiative between Emory University and Georgia Institute of Technology, are also working in this field. This field is highly competitive and companies and academic and research institutions are actively seeking to develop technologies that could be competitive with our technologies.
We are aware that other companies, organizations and persons have described technologies that appear to have some similarities to our patented proprietary technologies. In addition, academic institutions are also working in this field. Technological developments by others may result in our products and technologies, as well as products developed by our customers using our biocatalysts, becoming obsolete. We monitor publications and patents that relate to directed molecular evolution to be aware of developments in the field and evaluate appropriate courses of action in relation to these developments.
Many of our competitors have substantially greater manufacturing, financial, research and development, personnel and marketing resources than we do. In addition, certain of our competitors may also benefit from local government subsidies and other incentives that are not available to us. As a result, our competitors may be able to develop competing and/or superior technologies and processes, and compete more aggressively and sustain that competition over a longer period of time than we could. Our technologies and products may be rendered obsolete or uneconomical by technological advances or entirely different approaches developed by one or more of our competitors. As more companies develop new intellectual property in our markets, the possibility of a competitor acquiring patent or other rights that may limit our products or potential products increases, which could lead to litigation.
97
Table of Contents
We also face differing forms of competition in our various markets, as set forth below:
Pharmaceuticals
Our primary competitors in the pharmaceutical market are companies using conventional, non-biocatalytic processes to manufacture pharmaceutical intermediates and APIs that compete in the marketplace with our biocatalytically manufactured products. The principal methods of competition and competitive differentiation in this market are product quality and performance, including manufacturing yield and safety and environmental benefits, speed of delivery of product and price. The market for the manufacture and supply of APIs and intermediates is large with many established players. These companies include many of our large innovator and generic pharmaceutical customers, such as Merck, Pfizer, and Teva, who have significant internal research and development efforts directed at developing processes to manufacture APIs and intermediates. The processes used by these companies include classical conventional organic chemistry reactions, chemo catalysis reactions catalyzed by chemical catalysts, or biocatalytic routes using commercially available enzymes, or combinations thereof. Our manufacturing processes must compete with these internally developed routes. Additionally, there are many large well-established fine chemical manufacturing companies that compete to supply pharmaceutical intermediate and APIs to our customers, such as DSM, BASF Corporation and Lonza Group Ltd. Finally, we face increasing competition from generic pharmaceutical manufacturers in low cost centers such as India and China.
In addition to competition from companies manufacturing intermediates and APIs, we face competition from companies that sell biocatalysts for use in the pharmaceutical market. The market for supplying biocatalysts for use in pharmaceutical manufacturing is quite fragmented. There is competition from large industrial enzyme companies, such as Novozymes and Amano Enzyme Inc., whose industrial enzymes (for detergents, for example) are occasionally used in pharmaceutical processes. There is also competition in this area from several small European companies with relatively limited product offerings comprised primarily of naturally occurring biocatalysts. In addition to these biocatalyst supply companies, there is a separate group of small companies, also predominately in Europe, that offers biocatalyst optimization services.
We believe that our principal advantage is our ability to rapidly deliver customized biocatalyst products for existing and new APIs in the pharmaceuticals market. This capability has allowed us to create a breadth of product offerings with improved performance characteristics including, for example, activity, stability, and activity on a range of substrates, compared to traditional chemistry-based manufacturing processes and naturally occurring biocatalysts. We believe that our directed evolution technology provides substantially superior results, in shorter time frames, than companies offering competing biocatalyst development services.
Bioindustrials
There is increasing interest and activity in the bioindustrial market directed towards developing alternative manufacturing processes for products that have traditionally been derived from fossil fuel sources, such as transportation fuels and chemicals.
Currently, most biofuels being produced at commercial scale are ethanol derived from sugar and starch food sources, such as sugar cane and corn, and biodiesel produced from vegetable oils, such as soy oil. These markets are well-established with multiple companies, such as The Archer Daniels Midland Company, Cargill and a number of smaller companies producing ethanol in the United States.
Many established and several recently formed companies are developing biofuels technology and have forged relationships or ventures to develop and commercialize their technologies, including:
| | Novozymes, which has partnered with a number of companies and organizations on a regional basis to develop or produce biofuels, and recently opened a biofuel demonstration plant with Inbicon A/S of Denmark; |
98
Table of Contents
| | Danisco/Genencor, which has formed a joint venture with DuPont, called DuPont Danisco Cellulosic Ethanol, or DDCE, is marketing a line of cellulases to convert biomass into sugar; |
| | DSM, which received a grant from the U.S. Department of Energy to be the lead partner in a technical consortium including Abengoa Bioenergy New Technologies, is developing cost-effective enzyme technologies; |
| | Mascoma Corporation has entered into a feedstock processing and lignin supply agreement with Chevron Technology Ventures, a division of Chevron U.S.A., Inc.; and |
| | Verenium, which has entered into a research and development collaboration with BP, p.l.c and formed a joint venture with BP called Vercipia Biofuels to develop a commercial scale cellulosic ethanol facility. |
Although no company is currently converting cellulosic biomass into fermentable sugars at commercial scale, many of our competitors have been active in this area for many years, have invested significant resources in this effort, and have extensive patent portfolios regarding the relevant biocatalysts and related processes. In addition, several companies are focused on developing non-biocatalytic, thermochemical processes to convert cellulosic biomass into fermentable sugars. Our routes from cellulosic biomass to fermentable sugars will need to be cost-competitive with all of these alternative sources and routes. There are also many companies active in the area of producing non-ethanol biofuels from fermentable sugars. For example, DuPont has announced plans to develop and market biobutanol through Butamax, a joint venture with BP, while other companies such as Amyris Biotechnologies Inc., or Amyris, Gevo Inc. and LS9, Inc. are working on biocatalytic routes to non-ethanol biofuel alternatives to petroleum-based fuels. Virent Energy Systems and Shell also have a joint collaboration to develop theromochemical catalytic routes to biogasoline directly from sugars. Range Fuels Inc. is also focused on developing non-biocatalytic thermochemical processes to convert cellulosic biomass into fuels, and Coskata, Inc. is developing a hybrid thermochemical-biocatalytic process to produce ethanol from a variety of feedstocks. New companies are being founded in this area at an increasing rate. Many of these companies are actively developing and applying for intellectual property rights, including patent rights, in this space.
Our ability to remain competitive in this area will depend on our ongoing technical success in identifying and developing novel biocatalytic routes to fuel products that are cost-competitive not only with other biofuels but with petroleum-based fuels. Several of our competitors, including Amyris, utilize synthetic biology techniques to develop their products. Because these techniques have been in the public domain for many years, we are able to use these techniques together with our gene and genome directed evolution technologies. We believe that one of our principal advantages, particularly in the bioindustrial space, is that our directed evolution technology may enable us to develop new, more efficient, and therefore more cost-effective, biocatalysts and processes in less time than our competitors.
As we pursue opportunities in other bioindustrial markets, we expect to face competition from numerous companies focusing on developing biocatalytic and other solutions for these markets, including a number of the companies described above.
Operations
We conduct substantial operations outside of the United States. Please see Note 16 of our consolidated financial statements appearing elsewhere in this prospectus for a description of our revenues and long-lived assets outside of the United States. We have facilities located throughout the world, including in Redwood City, California, Singapore, and Budapest, Hungary. As of September 30, 2009, we employed 300 people worldwide, with 206 of our employees located in Redwood City.
Our corporate headquarters is located in Redwood City and provides general administrative support to our business and is the center of our manufacturing and research and development operations. In 2007, we
99
Table of Contents
established a research and development facility in Singapore to reduce our pharmaceutical research and development costs and to take advantage of the highly educated and skilled labor force in Singapore. In 2008, we established our facilities in Budapest, Hungary to create a research and development center for microbial biocatalyst improvement and fermentation development and to reduce our research and development costs. Hungary also has a highly educated and skilled work force that leverages the long history of fermentation development in Eastern Europe. Our facilities in Hungary are currently used exclusively for biofuels research and development.
Our research and development operations include efforts directed towards biocatalyst evolution, bioprocess development, cellular engineering, biocatalyst screening, metabolites, strain improvement, fermentation development and process engineering. We conduct enzyme evolution, enzyme production development, microbial bioprocess development, cellular engineering, microbial evolution and process engineering evaluations and design primarily at our headquarters in Redwood City. We also conduct biocatalyst evolution, biocatalyst screening and bioprocess development in Singapore. Our facility in Hungary collaborates with our headquarters in Redwood City in research and development activities relating to microbe improvement and is our center of excellence for strain and fermentation development. For more information on our research and development expenses, including expenses funded by our collaborative partners, see Managements Discussion and Analysis of Financial Condition and Results of Operations Revenues and Operating Expenses Research and Development Expenses included elsewhere in this prospectus.
We have limited internal manufacturing capacity at our headquarters in Redwood City. We expect to rely on third-party manufacturers for commercial production of our biocatalysts for the foreseeable future. Our in-house manufacturing is dedicated to producing both our Codex Biocatalyst Panels and biocatalysts for use by our customers in pilot scale production. We also supply initial commercial quantities of biocatalysts for use by our collaborators to produce pharmaceutical intermediates and manufacture biocatalysts that we sell.
We rely on two primary contract manufacturers, CPC Biotech srl, or CPC, and Lactosan GmbH & Co. KG, or Lactosan, to manufacture all of the commercial enzymes used in our pharmaceutical business. We have qualified other contract manufacturers to manufacture biocatalysts for our pharmaceutical business, but we do not currently rely on them for any of our supply requirements. We also rely on Arch, headquartered in Mumbai, India, to manufacture certain of our pharmaceutical intermediates and APIs as well as to provide sales and marketing support for these products in Asia, Latin America and the Middle East, and marketing support for these products in India, the United States, Canada, Europe and Israel. In addition, we contract with other suppliers in Austria, Germany, Italy and India.
We continue to evaluate whether to develop internal capabilities to manufacture biocatalysts at commercial scale. To increase our biocatalyst manufacturing capacity, we may invest in our own manufacturing capabilities through the construction of additional manufacturing facilities. The factors we will consider in deciding whether to expand our internal manufacturing capabilities include the costs associated with developing and maintaining such capabilities, the time required to develop such capabilities, potential locations for manufacturing sites, including proximity to existing customers, taxes associated with manufacturing activities and local incentives.
Facilities
Our headquarters is located in Redwood City, where we occupy approximately 87,000 square feet of office and laboratory space. The term of the lease expires in January 2011 for one part of our facilities, in April 2012 for another part and March 2013 for the third part. We have one option to extend the lease for an additional term of five years for each part, provided that we provide notice to the landlord at least nine months prior to the expiration of the initial term of the lease for each part. We believe that the facilities that we currently lease are adequate for our needs for the immediate future and that, should it be needed, additional space can be leased to accommodate any future growth.
100
Table of Contents
In Singapore, we occupy approximately 1,900 square meters of office and laboratory space within Singapore Science Park II. The term of the lease expires in July 2010. We have an option to extend the lease for an additional term of three years. We believe that the facilities that we currently lease in Singapore are adequate for our needs for the immediate future and that, should it be needed, additional space can be leased to accommodate any future growth.
In Hungary, we occupy approximately 900 square meters of office and laboratory space. The term of the lease expires in July 2013. We have an option to extend the lease for an additional term of five years. We believe that the facilities that we currently lease are adequate for our needs for the immediate future and that, should it be needed, additional space can be leased to accommodate any future growth.
Employees
As of September 30, 2009, we had 300 employees. Of these employees, 185 were engaged in research and development, 51 were engaged in manufacturing and operations, and 64 were engaged in general and administrative activities, respectively. We plan to continue to expand our research and development activities. To support this growth, we will need to expand managerial, research and development, operations, finance and other functions. None of our employees are represented by a labor union, and we consider our employee relations to be good.
Legal Proceedings
We are not currently a party to any material litigation or other material legal proceedings.
101
Table of Contents
Executive Officers, Key Employees and Directors
The following table sets forth certain information about our executive officers, key employees and directors, as of December 1, 2009.
| Name |
Age | Position |
||
| Executive Officers |
||||
| Alan Shaw |
46 | President and Chief Executive Officer, Director |
||
| Robert J. Lawson |
45 | Senior Vice President and Chief Financial Officer |
||
| David L. Anton |
56 | Senior Vice President, Research and Development |
||
| Joseph J. Sarret |
42 | Chief Business Officer and President, Pharmaceutical Services and Enzyme Products |
||
| Douglas T. Sheehy |
42 | Senior Vice President, General Counsel and Secretary |
||
| Key Employees |
||||
| John H. Grate |
57 | Senior Vice President, Science and Innovation and Chief Science Officer |
||
| Michael J. Knauf |
50 | Vice President and General Manager, Bioindustrials |
||
| Directors |
||||
| Thomas R. Baruch(1) (2) (3) |
71 | Chairman, Board of Directors |
||
| Alexander A. Karsner |
42 | Director |
||
| Bernard J. Kelley(1) (2) |
68 | Director |
||
| Bruce Pasternack(1) (3) |
62 | Director |
||
| Chris Streng |
43 | Director |
||
| James R. Sulat |
59 | Director |
||
| Dennis P. Wolf(2) (3) |
56 | Director |
||
| Mun Yew Wong |
38 | Director |
||
| (1) | Member of the Compensation Committee. |
| (2) | Member of the Audit Committee. |
| (3) | Member of the Nominating and Corporate Governance Committee. |
Alan Shaw, Ph.D., has served as President of Codexis since its inception and Chief Executive Officer since 2002. He has been a member of our board of directors since 2002. Prior to Codexis, Dr. Shaw was Head of New Business Development for Clariant and Managing Director for Lancaster Synthesis and prior to Clariants acquisition of BTP plc, Chief Operating Officer of Archimica, the pharmaceutical chemicals division of BTP plc. From 1994 to 1999, he was with Chiroscience Group plc, most recently as Managing Director of the pharmaceutical services unit, Chirotech Technology Limited, and a member of the board of directors of Chiroscience Ltd. Earlier in his career, Dr. Shaw held various scientific and management positions for over 15 years at Imperial Chemical Industries PLC (ICI)/Zeneca. Dr. Shaw serves on the board of directors of BIO, the biotechnology industry trade association, and is chair of the BIO Industrial and Environmental Section. He holds a B.S. in chemistry from Teesside University, England and a Ph.D. in chemistry from the University of Durham, England. Dr. Shaw is a Fellow of the Royal Society of Chemistry (FRSC, C.Chem.) and the Chartered Institute of Marketing (FCIM, Chartered Marketer).
102
Table of Contents
Robert J. Lawson has served as Senior Vice President and Chief Financial Officer since November 2009. Prior to joining Codexis, Mr. Lawson was most recently Vice President, Finance-Consumer Group of Intuit. While at Intuit from 2001 to November 2009, Mr. Lawson held various senior financial management positions, including Vice President, Investor Relations and Financial Planning and Analysis and Vice President, Finance-Small Business and Personal Finance. Prior to Intuit, Mr. Lawson served for 15 years in various financial management roles at General Electric. He holds a B.S. in business from Iowa State University.
David L. Anton, Ph.D., has served as Senior Vice President, Research and Development since May 2009. He joined Codexis in March 2008 as Vice President, Research and Development, for Codexis Bioindustrials. Dr. Anton has over 25 years experience directing development of new technology solutions and production processes. He joined DuPont in 1983, and held a variety of senior research management positions across bioprocessing and biocatalysis. He holds a B.S. in biochemistry from the University of California, Berkeley, and a Ph.D. in biochemistry from the University of Minnesota.
Joseph J. Sarret, M.D., J.D., has served as Chief Business Officer and President, Pharmaceutical Services and Enzyme Products since October 2009. He joined Codexis in 2005 as Corporate Counsel and Director, Business Development and was promoted to Vice President, Corporate Development in 2007 and Senior Vice President, Corporate Development in February 2009. Previously, he was an associate at Latham & Watkins LLP. He also served as attending physician and later Acting Medical Director for the HIV Clinic at the University of California, San Francisco Medical Center. Dr. Sarret is a graduate of both the University of California, San Francisco School of Medicine and Stanford Law School. He holds a B.A. in human biology from Stanford University, where he graduated Phi Beta Kappa.
Douglas T. Sheehy has served as Senior Vice President, General Counsel and Secretary of Codexis since November 2009. He joined Codexis in April 2007 as Vice President, General Counsel and Secretary. Prior to Codexis, Mr. Sheehy spent five years at CV Therapeutics, Inc. in various positions, most recently as Executive Director, Legal Corporate Law. Prior to that, Mr. Sheehy served as an attorney with the law firms of Gunderson Dettmer LLP and Brobeck Phleger & Harrison LLP. Mr. Sheehy holds a B.A. in history from Dartmouth College and a J.D. from American University.
John H. Grate, Ph.D., has served as Chief Science Officer and Senior Vice President, Science and Innovation since May 2009. From December 2007 to May 2009, Dr. Grate served as Chief Technology Officer and Senior Vice President, Technology and Innovation of Codexis. From July 2005 to December 2007, Dr. Grate served as Senior Vice President, Research and Development, and Chief Technology Officer of Codexis, and from September 2002 to July 2005, Dr. Grate served as Vice President, Research and Development and Chief Technology Officer. Prior to his employment with Codexis, Dr. Grate was an independent consultant and a member of Codexis Industrial Advisory Board. Previously, Dr. Grate held various research and development leadership positions in his 20 years at Catalytica, Inc. He was founding Vice President of Research and Development for the subsidiary, Catalytica Pharmaceuticals, Inc., until its acquisition by Royal DSM N.V. in early 2001. Dr. Grate is a registered U.S. Patent Agent. He holds a B.S. in chemistry from Miami University (Ohio) and a Ph.D. in chemistry from the University of California, San Diego.
Michael J. Knauf has served as Vice President and General Manager, Bioindustrials since April 2007. He joined Codexis from Lallemand Specialties, where he was General Manager of the Ethanol Technology business unit from June 2005 to March 2007. Previously, he served for nearly 20 years with Genencor, where he rose to Director and Industry Manager for Fermentation Alcohol Enzymes. Mr. Knauf holds a B.S in biochemistry and biophysics and a masters degree in food science from the University of California, Davis.
Thomas R. Baruch has served as a director of Codexis since 2002. Mr. Baruch is the founder and a managing director of CMEA Ventures, a venture capital firm that was established in 1989 as an affiliated fund of New Enterprise Associates. Mr. Baruch is currently on the board of directors of Entropic
103
Table of Contents
Communications, Inc., and several private companies. Before starting CMEA Ventures, Mr. Baruch was a founder and Chief Executive Officer of Microwave Technology, Inc., a supplier of gallium arsenide integrated circuits. Prior to his employment with Microwave Technology, Inc., Mr. Baruch managed a dedicated venture fund at Exxon Corp, and was president of the Exxon Materials Division. Earlier in his career, Mr. Baruch worked as a patent attorney and remains a registered patent attorney. He is also both a member of the Executive Committee of the Council of Competitiveness and a member of the Steering Committee of the ESIS Initiative (Energy, Security, Innovation and Sustainability) of the Council of Competitiveness. Mr. Baruch is a member of the board of trustees of Rensselaer Polytechnic Institute and the board of trustees of the Berkeley Institute of Synthetic Biology. Mr. Baruch holds a B.S. in engineering from Rensselaer Polytechnic Institute and a J.D. from Capital University.
Alexander A. Karsner has served as a director of Codexis since December 2009. He is currently Chief Executive Officer of Manifest Energy, LLC, a clean energy infrastructure development and finance company. Mr. Karsner served as Assistant Secretary for Energy Efficiency and Renewable Energy at the U.S. Department of Energy from March 2006 to August 2008. From April 2002 to March 2006, Mr. Karsner was Managing Director of Enercorp LLC, a private company involved in international project development, management and financing of renewable energy infrastructure. Mr. Karsner has also worked with Tondu Energy Systems of Texas, Wartsila Power Development of Finland and other multi-national energy firms and developers. Mr. Karsner is a director of Applied Materials, Inc., Conservation International, Argonne National Laboratory, the Gas Technology Institute, the National Marine Sanctuaries Foundation and is on the advisory board of Hudson Clean Energy and the Automotive X Prize. He is a Distinguished Fellow at the Council on Competitiveness and a leader of the Energy Future Coalition. Mr. Karsner earned a Masters degree at Hong Kong University and a Bachelors degree with honors from Rice University.
Bernard J. Kelley has served as a director of Codexis since April 2004. From 1993 to 2002, Mr. Kelley was the President of the Merck Manufacturing Division, a division of Merck & Co., Inc., and he served as a member of the Merck Management Committee from 1995 to 2002. Mr. Kelley currently serves on the board of directors, compensation and audit committees of MAP Pharmaceuticals, Inc., a biotechnology company, and previously served on the board of directors of Aegis Analytical Corporation, an enterprise software company, from 2004 to 2006. He holds a B.S. in engineering from the U.S. Naval Academy.
Bruce Pasternack has served as a director of Codexis since August 2007. Mr. Pasternack is currently a venture partner of CMEA Capital. From June 2005 to May 2007, Mr. Pasternack served as the President and Chief Executive Officer of Special Olympics, Inc. Prior to his employment with Special Olympics, Inc., Mr. Pasternack spent more than 28 years at Booz Allen Hamilton Inc., where his last position was Senior Vice President and Managing Partner of its San Francisco office. From 1973 to 1976, he served as Associate Administrator for Policy and Program Evaluation at the Federal Energy Administration, and Staff Director of the Presidents Energy Resources Council. From 1972 to 1973, he served on the staff of the Presidents Council on Environmental Quality in the Executive Office of the President. From 1968 to 1972, he was a systems engineer at General Electric. Mr. Pasternack is a director of Quantum Corporation and Symyx Technologies, Inc., a member of the board of trustees of The Cooper Union and has previously served on the board of directors of BEA Systems, Inc. and the Special Olympics, Inc. At Symyx Technologies, he is Lead Director and Chairman of the Compensation Committee. At Quantum Corporation, he is a member of the Compensation Committee. He holds a B.E. from The Cooper Union and a M.S.E. from the University of Pennsylvania.
Chris Streng has served as a director of Codexis since March 2009. He is currently employed by Shell Downstream Inc., an affiliate of Royal Dutch Shell plc and its affiliated companies, or the Shell Group, where he has served as Vice President Finance Manufacturing since 2007 and is based in Houston, Texas. In such position, he is responsible for finance for refinery and petrochemical plants in the Shell Group
104
Table of Contents
worldwide. From 2005 to 2007, Mr. Streng was Vice President Group Planning & Appraisal, based in The Hague, The Netherlands. He joined the Shell Group in 1990, and has held financial management positions in the Shell Groups exploration and production, refining and chemicals businesses, as well as the mergers & acquisitions and treasury functions in The Netherlands, the United Kingdom, Norway and the United States. He also serves as a director or in an equivalent position for certain refining joint ventures in which Shell Group companies are owners. Mr. Streng holds a masters degree in finance from the London Business School and graduated in business engineering from the University of Twente, The Netherlands.
James R. Sulat has served as a director of Codexis since October 2009. He was named Chief Executive Officer and Chief Financial Officer of Maxygen in 2009. He has served as a director of Maxygen since 2003. He served as Chief Financial Officer of Memory Pharmaceuticals Corp., a biotechnology company in 2008, and Chief Executive Officer from 2005 to 2008. Mr. Sulat was Senior Executive Vice President and Interim Chief Financial Officer of R.R. Donnelley & Sons Co., a diversified printing company, from February 2004 until May 2004. From April 2003 to February 2004, Mr. Sulat was Senior Executive Vice President of Moore Wallace Incorporated, a diversified printing company that was acquired by R.R. Donnelley in 2004. From April 1998 to April 2003, Mr. Sulat was Vice President and Chief Financial Officer of Chiron Corporation, a biotechnology company. Mr. Sulat is also a director of Momenta Pharmaceuticals, Inc., a publicly-traded biotechnology company focused on the development of protein pharmaceuticals, and Intercell AG, a developer of vaccines for the prevention and treatment of major infectious diseases that is listed on the Vienna Stock Exchange. Mr. Sulat holds a B.S. from Yale University, an M.B.A. from Stanford University and an M.S. in health services administration from Stanford University.
Dennis P. Wolf has served as a director of Codexis since December 2007. Mr. Wolf serves as Senior Vice President and Chief Financial Officer of Fusion-io Multisystems. Previously, Mr. Wolf served as Executive Vice President and CFO of MySQL AB. Prior to MySQL, Mr. Wolf held financial management positions for public high technology companies including Apple Computer, Inc., Centigram Communications, Inc., Credence Systems Corporation, Omnicell, Inc., Redback Networks Inc. and Sun Microsystems, Inc. Mr. Wolf is a director of Bigband Networks and Quantum Corporation, and has been a director and chair of the audit committee for public companies including Avanex Corporation, Komag, Inc. and Vitria Technology, Inc. He holds a B.A. from the University of Colorado and an M.B.A. from the University of Denver.
Mun Yew Wong, M.D., has served as a director of Codexis since October 2009. He is Director (Investments), San Francisco Centre for EDB Investments Pte Ltd, or EDB Investments, and Bio*One Capital Pte Ltd, or Bio*One. Dr. Wong is experienced in clinical medicine as a family physician, and was a founder of a number of start-up companies in the fields of education, healthcare and medical tourism prior to joining Bio*One in 2005. He has served on boards of Bio*One portfolio companies NeuroVision Pte Ltd, KOOPrime Pte Ltd in Singapore and Amaranth Medical Inc. in the U.S. In February 2007, he was appointed as Director (Investments) at Bio*Ones U.S. office in the San Francisco Bay Area, focusing on the biomedical sciences sector. In addition to his role at Codexis, he is a board observer for Fluidigm Corporation, Pelikan Technologies, Inc., Kalobios Pharmaceuticals Inc., Broncus Technologies, Inc., Adamas Pharmaceuticals, Inc., Panomics, Inc. (acquired by Affymetrix), Innovalight, Inc. and Revance Therapeutics, Inc. He has held a concurrent appointment as Director (Investments) at EDB Investments since January 2009, expanding his portfolio coverage to the clean technologies and digital media sectors in the United States. He holds an M.D. from the National University of Singapore.
Board Composition
Our board of directors may establish the authorized number of directors from time to time by resolution. Ten directors are authorized and we currently have nine directors, of which five are designated by the current holders of our preferred stock, three are designated by the current holders of our preferred and common stock, and one also serves as our Chief Executive Officer. Dr. Wong and Mr. Sulat will resign
105
Table of Contents
from our board of directors in connection with the closing of our initial public offering. Of the members of our board of directors, Messrs. Baruch, Kelley, Pasternack, Wolf and Dr. Wong are independent directors as defined under the applicable rules and regulations of the Securities and Exchange Commission, or the SEC, and The Nasdaq Stock Market.
Under the terms of our amended and restated certificate of incorporation and the voting agreement among us and the holders of our preferred stock, the members of our board of directors are to be designated as follows: Equilon Enterprises LLC dba Shell Oil Products US, or Shell, has the right to designate two members; Biomedical Sciences Investment Fund Pte Ltd, CMEA Ventures Life Sciences 2000, L.P., FirstMark III, L.P. and Maxygen, Inc., each have the right to designate one member; one member shall be our Chief Executive Officer; and the remainder shall be designated with the consent of the parties holding a majority of the outstanding common and preferred stock. Upon the consummation of this offering, all of these provisions will terminate, except that for a ten-year period Shell will have the right to designate one board member for so long as: Shell holds at least 50% of the total number of shares of common stock issued upon conversion of the preferred stock purchased by Shell, and at least 5% of our fully diluted number of shares of common stock outstanding, and the collaborative research agreement between us and Shell has not expired or been terminated. The designee of Shell will be subject to the reasonable approval of a majority of the members of the board of directors.
In accordance with our amended and restated certificate of incorporation to take effect following the completion of this offering, our board of directors will be divided into three classes with staggered three-year terms. At each annual meeting of stockholders, the successors to directors whose terms then expire will be elected to serve from the time of election and qualification until the third annual meeting following election. After the completion of this offering, our directors will be divided among the three classes as follows:
| | the Class I directors will be , and , and their terms will expire at the annual meeting of stockholders to be held in 2011; |
| | the Class II directors will be , and , and their terms will expire at the annual meeting of stockholders to be held in 2012; and |
| | the Class III directors will be , and , and their terms will expire at the annual meeting of stockholders to be held in 2013. |
Our amended and restated certificate of incorporation will provide that the authorized number of directors may be changed only by resolution of the board of directors. Any additional directorships resulting from an increase in the number of directors will be distributed among the three classes so that, as nearly as possible, each class will consists of one-third of the directors. The division of our board of directors into three classes with staggered three-year terms may delay or prevent a change of our management or a change of control at our company.
Board Committees
Our board of directors has the following committees: an audit committee, a compensation committee and a nominating and corporate governance committee. The composition and responsibilities of each committee are described below. Members serve on these committees until their resignation or until otherwise determined by our board of directors.
Audit Committee
Our audit committee oversees our corporate accounting and financial reporting process. Among other matters, the audit committee appoints the independent registered public accounting firm; evaluates the
106
Table of Contents
independent registered public accounting firms qualifications, independence and performance; determines the engagement of the independent registered public accounting firm; reviews and approves the scope of the annual audit and the audit fee; discusses with management and the independent registered public accounting firm the results of the annual audit and the review of our quarterly consolidated financial statements; approves the retention of the independent registered public accounting firm to perform any proposed permissible non-audit services; monitors the rotation of partners of the independent registered public accounting firm on our engagement team as required by law; reviews our consolidated financial statements and our managements discussion and analysis of financial condition and results of operations to be included in our annual and quarterly reports to be filed with the SEC, reviews our critical accounting policies and estimates; and annually reviews the audit committee charter and the committees performance. The current members of our audit committee are Thomas R. Baruch, Bernard J. Kelley and Dennis P. Wolf. Mr. Wolf serves as the chairman of the committee. All members of our audit committee meet the requirements for financial literacy under the applicable rules and regulations of the SEC and The Nasdaq Stock Market. Our board of directors has determined that Mr. Wolf is an audit committee financial expert as defined under the applicable rules of the SEC and has the requisite financial sophistication as defined under the applicable rules and regulations of The Nasdaq Stock Market. Each of the members of our audit committee, except Mr. Baruch, qualifies as an independent director under the applicable rules and regulations of the SEC and The Nasdaq Stock Market relating to audit committee independence. Within one year from the date of effectiveness of our initial public offering registration statement, our board of directors intends to replace Mr. Baruch as a member of our audit committee with a person who will meet these heightened independence standards. The audit committee operates under a written charter that satisfies the applicable standards of the SEC and The Nasdaq Stock Market.
Compensation Committee
Our compensation committee reviews and recommends policies relating to compensation and benefits of our officers and employees. The compensation committee reviews and approves corporate goals and objectives relevant to compensation of our Chief Executive Officer and other executive officers, evaluates the performance of these officers in light of those goals and objectives, and sets the compensation of these officers based on such evaluations. The compensation committee also recommends to our board of directors the issuance of stock options and other awards under our stock plans. The compensation committee will review and evaluate, at least annually, the performance of the compensation committee and its members, including compliance of the compensation committee with its charter. The current members of our compensation committee are Thomas R. Baruch, Bernard J. Kelley and Bruce Pasternack. Mr. Pasternack serves as the chairman of the committee. Each of the members of our compensation committee is an independent or outside director under the applicable rules and regulations of the SEC, The Nasdaq Stock Market and the Internal Revenue Code of 1986, as amended, relating to Compensation Committee independence. The compensation committee operates under a written charter.
Nominating and Corporate Governance Committee
The nominating and corporate governance committee is responsible for making recommendations to our board of directors regarding candidates for directorships and the size and composition of our board of directors. In addition, the nominating and corporate governance committee is responsible for overseeing our corporate governance policies and reporting and making recommendations to our board of directors concerning governance matters. The current members of our nominating and corporate governance committee are Thomas R. Baruch, Bruce Pasternack and Dennis P. Wolf. Mr. Baruch serves as the chairman of the committee. Each of the members of our nominating and corporate governance committee is an independent director under the applicable rules and regulations of the SEC and The Nasdaq Stock Market relating to nominating and corporate governance committee independence. The nominating and corporate governance committee operates under a written charter.
107
Table of Contents
There are no family relationships among any of our directors or executive officers.
Compensation Committee Interlocks and Insider Participation
None of the members of our compensation committee has at any time during the prior three years been an officer or employee of ours. None of our executive officers currently serves or in the prior three years has served as a member of the board of directors or compensation committee of any entity that has one or more executive officers serving on our board of directors or compensation committee.
Code of Business Conduct and Ethics
We will adopt a code of business conduct and ethics that applies to all of our employees, officers and directors, including those officers responsible for financial reporting. The code of business conduct and ethics will be available on our website at www.codexis.com. We expect that any amendments to the code, or any waivers of its requirements, will be disclosed on our website.
Director Compensation
In June 2007, our board of directors adopted an Independent Director Compensation Plan pursuant to which those directors designated as directors who are not affiliated with the Companys major stockholders by the board of directors for purposes of the Independent Director Compensation Plan were entitled to receive an annual cash retainer of $35,000, paid in semi-annual installments on June 30 and December 31 of each year, and the reimbursement of any actual out-of-pocket expenses. In addition, the Independent Director Compensation Plan provides for the grant of an annual option to purchase 25,000 shares of our common stock, to be granted at the first board of directors meeting of each year. These options vest as to 1/4th of the total number of shares subject to the option on the first anniversary of the vesting commencement date, and 1/48th of the total number of shares subject to the option monthly thereafter until all shares are vested, subject to the continued service of the director on the board of directors. Pursuant to the Independent Director Compensation Plan, each of Messrs. Kelley, Pasternack and Wolf were granted an option to purchase 25,000 shares of our common stock on June 2, 2009 with a per share exercise price of $4.97, which our board of directors determined was the per share fair market value of our common stock as of the date of grant.
Following the completion of this offering, each non-employee director shall receive an annual cash retainer of $35,000 per year. Such directors shall also receive an additional annual cash retainer of $5,000 per year for being a member of our compensation committee, except that the chairperson of our compensation committee shall receive an additional annual cash retainer of $10,000 per year. Non-employee directors shall also receive an additional annual cash retainer of $4,000 per year for being a member of our nominating and corporate governance committee, except that the chairperson of our nominating and corporate governance committee shall receive an additional annual cash retainer of $8,000 per year. Non-employee directors shall also receive an additional annual cash retainer of $7,500 per year for being a member of our audit committee, except that the chairperson of our audit committee shall receive an additional annual cash retainer of $15,000 per year.
Upon election to our board of directors, each non-employee director shall receive an initial option grant of an option to purchase 20,000 shares of our common stock with a per share exercise price equal to the per share closing trading price of our common stock on the date of grant. Such initial option grant shall be vested and become exercisable as to 1/4th of the total number of shares subject to the option on the first anniversary of the date the director commences service on our board of directors, with the remainder of the option vesting and becoming exercisable at a rate of 1/48th of the total number of shares subject to the option each month thereafter. On the date of each annual meeting of stockholders beginning in 2010, each non-employee director who has served at least six months on our board of directors shall also receive an annual grant of an option to purchase 12,000 shares of our common stock with a per share exercise price
108
Table of Contents
equal to the per share closing trading price of our common stock on the date of grant. Such annual option grant shall be vested and become exercisable as to one twelfth of the total number of shares subject to the option on each monthly anniversary of the date of grant.
From August 2009, after the termination of employment of our former Chief Financial Officer, until October 31, 2009, Mr. Wolf provided additional services as chairman of the audit committee. Mr. Wolf received $5,000 per week for these additional services, which were limited to advising management on accounting and financial matters.
On December 14, 2009, we entered into a consulting agreement with Mr. Karsner pursuant to which he agreed to provide strategic advisory services related to the energy industry and government policy in connection with our proprietary enzyme and biocatalytic processes. Pursuant to the terms of the agreement, Mr. Karsner is entitled to receive, in his capacity as a consultant, $30,000 per quarter and was granted stock options to purchase 100,000 shares of our common stock at an exercise prices of $6.06 per share, which our directors determined was the per share fair market value of our common stock as of the date of the grant. These options vest at a rate of 1/48th of the total shares subject to the option each month from the date of the agreement, subject to Mr. Karsners continued service as a consultant. On December 14, 2009, pursuant to the Independent Director Compensation Plan, Mr. Karsner was also granted an option to purchase 25,000 shares of our common stock, also with a per share exercise price of $6.06.
Director Compensation Table
The following table sets forth information regarding compensation earned by our non-employee directors during the fiscal year ended December 31, 2009.
| Name |
Fees Earned or Paid in Cash ($) |
Option Awards ($)(1) |
All Other Compensation ($) |
Total ($) |
||||||||||
| Thomas R. Baruch |
$ | | $ | | $ | | $ | | ||||||
| Bernard J. Kelley |
35,000 | 83,385 | | 118,385 | ||||||||||
| Bruce Pasternack |
35,000 | 83,385 | | 118,385 | ||||||||||
| Dennis P. Wolf |
35,000 | 83,385 | 88,000 | (2) | 206,385 | |||||||||
| Chris Streng |
| | | | ||||||||||
| Mun Yew Mong, M.D. |
| | | | ||||||||||
| James R. Sulat |
| | | | ||||||||||
| Alexander A. Karsner |
1,630 | 625,763 | (3) | | 627,393 | |||||||||
| (1) | Amount reflects the grant date fair value of options granted in the year ended December 31, 2009 calculated in accordance with Statement of Financial Accounting Standard Board Accounting Standards Codification Topic 718, Stock Compensation, or ASC Topic 718, other than as set forth in footnote 3. The valuation assumptions used in determining such amounts are described in Note 12 to our financial statements included in this prospectus. As of December 31, 2009, Mr. Kelley, Mr. Pasternack, Mr. Wolf and Mr. Karsner had outstanding option awards to purchase an aggregate of 107,500, 75,000, 75,000 and 125,000 shares, respectively. |
| (2) | Amount includes fees earned for additional services as chairman of the audit committee, which were limited to advising management on accounting and financial matters after the termination of employment of our former Chief Financial Officer on June 30, 2009 until October 31, 2009. |
| (3) | $525,580 of such amount reflects the grant date fair value of options to purchase 100,000 shares of our common stock granted to Mr. Karsner on December 14, 2009 in consideration of his service as a consultant to us, as calculated in accordance with Statement of Financial Accounting Standard Board Accounting Standards Codification Topic 505.50, Equity-Based Payments to Non-Employees, or ASC Topic 505.50. The remaining $100,183 is the grant date fair value for options granted to |
109
Table of Contents
| Mr. Karsner as a director, also calculated in accordance with ASC Topic 718. The valuation assumptions used in determining such amount are similar to the assumptions described in Note 12 to our financial statements included in this prospectus. |
Executive Compensation
Compensation Discussion and Analysis
Our executive compensation program is designed to attract talented individuals to lead, manage and operate all aspects of our business and reward and retain those individuals who continue to meet our high expectations over time. Our executive compensation program combines short- and long-term components, cash and equity, and fixed and contingent payments in the amounts and proportions that we believe are most appropriate to incentivize and reward our executive officers for achieving our objectives. Our executive compensation program also is intended to make us competitive in our industry, where there is considerable competition for talented executives.
Our named executive officers for fiscal year 2009 were Alan Shaw, Ph.D., President and Chief Executive Officer; Robert J. Lawson, Senior Vice President and Chief Financial Officer; Joseph J. Sarret, M.D., J.D., Chief Business Officer and President, Pharmaceutical Services and Enzyme Products; Douglas T. Sheehy, Senior Vice President, General Counsel and Secretary; David L. Anton, Ph.D., Senior Vice President, Research and Development; and Robert S. Breuil, former Senior Vice President, Finance and Chief Financial Officer. Mr. Breuils employment with us terminated as of June 30, 2009.
Objectives and Philosophy of Our Executive Compensation Program
Our compensation program for our named executive officers is designed to achieve the following objectives:
| | attract, engage and retain individuals of superior ability, experience and managerial talent enabling us to be an employer of choice in our highly-competitive and dynamic industry; |
| | motivate and reward executives whose knowledge, skills and performance ensure our continued success; |
| | encourage and inspire our executives to achieve key corporate performance objectives by linking base salary increases and incentive award opportunities to the achievement of individual and company-wide short- and long-term goals; and |
| | align the interests of our executives and stockholders by motivating executives to increase stockholder value, by providing a significant portion of total compensation opportunities for our executive officers in the form of direct ownership in our company through stock options and other equity awards. |
Components of Our Executive Compensation Program
The components of our executive compensation program consist primarily of base salaries, annual cash incentive bonuses, equity awards and broad-based benefits programs. We combine short-term compensation components (such as base salaries and annual cash incentive bonuses) and long-term compensation components (such as equity incentive awards) to provide an overall compensation structure that is designed to both attract and retain key executives as well as provide incentive for the achievement of short- and long-term corporate objectives.
The compensation committee of our board of directors is responsible for evaluating and administering our compensation programs and practices for our executive officers. Our compensation committee uses its judgment and experience and the recommendations of the Chief Executive Officer to determine the
110
Table of Contents
appropriate mix of short- and long-term compensation elements for each named executive officer. Short- and long-term compensation elements are balanced to encourage each executive officer to use his or her time and talents to accomplish both our short- and long-term corporate objectives. Our Chief Executive Officer, General Counsel and Vice President of Human Resources each attend our compensation committee meetings to provide input on factors that may influence our compensation committee members consideration of compensation programs and individual compensation, including individual performance, financial, legal and compensation parity considerations. In addition, our Chief Financial Officer occasionally attends such compensation committee meetings depending on the issues being discussed. Each such officer is not present at the meetings at the time that his or her own compensation is being reviewed by the committee. Our compensation committee analyzes each of the primary elements of our compensation program to ensure that our executives overall compensation is competitive with executive officers in similar positions at comparable companies in our labor market and to ensure internal compensation parity among our executive officers. Our compensation committee recommends and our board of directors approves equity incentive awards for our employees, including our executive officers.
Our compensation committee determines compensation for our executive officers, including our named executive officers, in large part based upon our financial resources, as well as competitive market data. With regard to annual base salaries and annual cash incentive bonus opportunity targets for fiscal year 2009, our compensation committee reviewed comprehensive compensation data from the Radford Global Life Sciences Survey, which aggregated survey results from 130 biotechnology, pharmaceutical and medical device companies in Northern California with revenues of less than $1 billion. For fiscal year 2009, our compensation committee also reviewed data aggregated and compiled by Compensia, Inc. from a late 2008 survey of a large number of late-stage, pre-IPO life sciences companies. For the purposes of the Compensia survey, late-stage was defined as companies which had raised more than $75 million in capital. While our compensation committee reviewed compensation information from the Radford and Compensia surveys, our compensation committee was not aware of the identity of the surveyed companies and, as such, did not rely on data for any single company.
In late September 2009, based on the recommendation of Compensia, our compensation committee adopted a peer group of companies, which expands beyond life sciences companies and includes public biotechnology, biofuels/chemical and clean technology companies. The peer group for 2010 includes the following companies:
| Affymax Inc. Dionex Corporation Energy Recovery, Inc. Evergreen Energy Inc. Exelixis Inc. FuelCell Energy, Inc. Genomic Health, Inc. InterMune, Inc. Luminex Corporation |
Martek Biosciences Corporation Maxygen, Inc. Metabolix, Inc. Rentech, Inc. SurModics, Inc. Symyx Technologies, Inc. Verenium Corporation XenoPort, Inc. |
We believe that the practices of the companies in the surveys we reviewed provide us with appropriate compensation benchmarks because many of these companies have similar organizational structures and tend to compete with us for executives. We work within the general framework of this market-competitive philosophy to determine each component of an executives compensation package based on numerous factors, including:
| | the demand for the particular skill sets we need within the marketplace; |
| | performance goals and other expectations for the position and the individual; |
111
Table of Contents
| | the individuals background and relevant expertise, including training and prior relevant work experience; |
| | the individuals role with us and the compensation paid to similar persons at the companies that participate in the surveys that we review; and |
| | comparison to other executives within our company having similar levels of expertise and experience. |
During 2009, our compensation committee reviewed all aspects of our executive compensation program, including base salaries, annual cash incentive bonuses and equity incentive targets for each of our executive officers. To ensure that top talent could be retained and attracted, in 2009 the compensation committee approved adjustments to our executive compensation program to reflect competitive pressures and ensure internal equity among executives with similar levels of responsibility and authority.
Each of the primary elements of our executive compensation program is discussed in more detail below. While we have identified particular compensation objectives that each element of executive compensation serves, our compensation programs are designed to be flexible and complementary and to collectively serve all of the executive compensation objectives described above. Accordingly, whether or not specifically mentioned below, we believe that, as a part of our overall executive compensation policy, each individual element of our executive compensation program, to a greater or lesser extent, serves each of our objectives as set forth above.
Annual Cash Compensation
Base Salary
The base salaries of all executive officers are reviewed annually and adjusted when necessary to reflect individual roles and performance, and the competitive market. Our compensation committee also reviews each executives annual base salary in comparison with other executives who are at the same level at our company and seeks parity among executives with similar levels of responsibility and authority. Our compensation committee believes that a competitive base salary is a necessary element of any compensation program designed to attract and retain talented and experienced executives. We also believe that competitive base salaries can motivate and reward executives for their overall performance.
However, in February 2009, in light of the then current economic conditions, our compensation committee decided to freeze all employees salaries, including our named executive officers, at their 2008 levels, with the exception of increases due to promotions and adjustments for exceptional performance for those employees who had base salaries which fell below the 50th percentile of the companies in our Peer Group for similar positions. The salary freeze was implemented in light of then-current economic conditions, similar salary freezes taking place at other similar companies in our geographical area and in order to preserve our cash reserves in the face of uncertainty in the financial and credit markets. In February 2009, upon recommendation of our Chief Executive Officer, after determining that Mr. Sheehy had exhibited exceptional performance in 2008 and was paid below the 50th percentile of executives, the compensation committee increased his base salary by $10,000 to $270,000. In November 2009, our compensation committee increased Mr. Sheehys base salary from $270,000 to $300,000 in connection with his promotion to Senior Vice President, General Counsel and Secretary. Our compensation committee increased Dr. Antons base salary from $235,000 to $250,000 in February 2009 and to $270,000 in May 2009 in connection with promotions. Dr. Anton currently serves as Senior Vice President, Research and Development. Our compensation committee also increased Dr. Sarrets base salary from $240,000 to $270,000 in February 2009 and to $320,000 in October 2009 in connection with promotions. Dr. Sarret currently serves as Chief Business Officer and President, Pharmaceutical Services and Enzyme Products. The following table sets forth the base salaries for 2009 for each of our named executive officers and,
112
Table of Contents
where applicable, the percentage such salary increased over such executives base salary as of December 31, 2008:
| Name of Executive Officer |
Increase | 2009 Base Salary Rate | ||||
| Alan Shaw, Ph.D. |
| % | $ | 425,000 | ||
| Robert J. Lawson |
| 330,000 | ||||
| Douglas T. Sheehy |
15.4 | 300,000 | ||||
| David L. Anton, Ph.D. |
14.9 | 270,000 | ||||
| Joseph J. Sarret, M.D., J.D. |
33.3 | 320,000 | ||||
| Robert S. Breuil |
| 320,000 | ||||
Annual Cash Incentive Bonuses
Our compensation philosophy with respect to annual cash incentive bonuses is consistent with our overall compensation program philosophy. The annual cash incentive bonus is directed at tying individual compensation to both corporate and individual performance while maintaining market-competitive compensation. Performance, as measured against individual and corporate goals, directly affects the level of bonus payment.
Annual Cash Incentive Bonuses for 2009
In June 2009, our compensation committee adopted the 2009 Executive Incentive Compensation Plan, under which the annual cash incentive bonus targets set forth below were used along with corporate and individual performance targets set by our compensation committee.
For 2009, our compensation committee retained the same target bonus percentages as in 2008 for Dr. Shaw and Mr. Breuil. Dr. Antons bonus target percentage was increased to 30% of his base salary in February 2009 and to 40% of his base salary in May 2009, in connection with promotions. He currently serves as Senior Vice President, Research and Development. Likewise, Mr. Sheehys target bonus percentage was increased to 40% in connection with his promotion to Senior Vice President, General Counsel and Secretary, which took place in November 2009. Similarly, Dr. Sarrets target bonus percentage was increased to 40% in February 2009 in connection with his promotion to Senior Vice President, Corporate Development. He currently serves as our Chief Business Officer and President, Pharmaceutical Services and Enzyme Products. In setting Dr. Antons, Mr. Sheehys and Dr. Sarrets target bonus percentage, our compensation committee considered the target bonus percentages of executives having a similar level of responsibility within our company and data from the surveys we reviewed. Mr. Lawson was not eligible for a bonus in 2009, as he joined our company in November 2009 and the 2009 Executive Incentive Compensation Plan does not permit participation for those who join the company after October 1, 2009. The table below sets forth the annual cash incentive bonus target for each of our named executive officers who was eligible to receive a bonus in 2009:
| Name of Executive Officer |
2009 Bonus Target (as % of 2009 Base Salary) |
||
| Alan Shaw, Ph.D. |
50 | % | |
| Douglas T. Sheehy(1) |
31 | ||
| David L. Anton, Ph.D.(2) |
36 | ||
| Joseph J. Sarret, M.D., J.D.(3) |
38 |
| (1) | Represents a prorated amount. Mr. Sheehys bonus target percentage was increased from 30% to 40% in November 2009 in connection with his promotion to Senior Vice President, General Counsel and Secretary. |
| (2) | Represents a prorated amount. Dr. Antons bonus target percentage was increased first from 25% to 30% in February 2009 in connection with his promotion to Vice President Level II, Bioindustrial |
113
Table of Contents
| Research and Development, and then from 30% to 40% in May 2009 in connection with his promotion to Senior Vice President, Research and Development. |
| (3) | Represents a prorated amount. Dr. Sarrets bonus target percentage was increased from 30% to 40% in February 2009 in connection with his promotion to Senior Vice President, Corporate Development. |
The company performance factor is subdivided into two separate factors: (i) the company non-financial performance factor; and (ii) the company financial performance factor. The company financial performance factor is measured based upon our companys achievement of three equally weighted financial goals established by our compensation committee, relating to net revenues, earnings before the deduction of interest, tax, depreciation and amortization, or EBITDA, and year-end cash (book value of unrestricted cash and securities). The non-financial performance goals that comprise the company non-financial performance factor include the achievement of certain milestones related to the achievement of our strategic plan and improving internal controls. The company financial performance factor represents 45% of the total company performance factor and the company non-financial performance factor represents the other 55%. The company financial performance factor targets for net revenue, EBITDA and year-end cash for 2009 were $81.6 million, $(9.1) million and $37.0 million, respectively.
The individual performance factor of the bonus is measured by our Chief Executive Officers, or in the case of our Chief Executive Officers performance, our compensation committees, assessment of the overall performance of each of our executives using individual goals established for each executive by our compensation committee. These individual goals, and the target bonus percentages, are established based on our Chief Executive Officers and our compensation committees evaluation of each executives position within the company, the corporate goals over which that executive has control or influence and the market practices of the companies in the surveys we reviewed. In setting individual performance factors and target bonus percentages for our named executive officers, our Chief Executive Officer, or in the case of our Chief Executive Officers factor and target, our compensation committee also considered the target bonus percentages and individual performance factors of executives with similar levels of responsibility within the company to ensure parity between executives at similar position levels. The individual goals that comprise the individual performance factor for any one named executive officer are too numerous for any single individual goal to have a material impact on a named executive officers total compensation but, taken as a whole, provide our Chief Executive Officer and our compensation committee insight into the individual performance level of our named executive officers. Examples of individual goals include achieving departmental budgets for revenues and margin, meeting sales and/or testing objectives, achieving milestones related to the development of new products, achieving recognition for a product or facility, securing supplies, meeting expansion goals and achieving or maintaining a professional standard. The individual goals that comprise the individual performance factor are set to be difficult to achieve and require above what our compensation committee has determined to be average performance in order to meet the minimum standard. Achievement against the goals set by the compensation committee or the Chief Executive Officer is determined by assessing whether a majority of individual goals were met or exceeded and is subject to upward and downward discretion by the Chief Executive Officer or the compensation committee.
Under the 2009 Executive Incentive Compensation Plan, no bonus is payable if our company achieves less than 80% of any single company financial performance goal, or if the executives achievement of his individual target is less than 80%. Failure to achieve 80% of any goal that comprises the company non-financial performance factor will result in a zero for that particular goal, but will not alone result in zero total bonus. The maximum company performance factor achievement level is 120%, and there is a direct correlation between actual achievement and the company performance factor. Similarly, the maximum individual performance factor achievement level is 150%, with a direct correlation between individual achievement and the individual performance factor as follows:
Bonus Amount = (Base Salary) x (Target Percentage) x ((.45 x Company Financial Performance Factor) + (.55 x Company Non-Financial Performance Factor)) x (Individual Performance Factor)
114
Table of Contents
Achievement levels of our companys corporate financial performance and non-financial performance factors, as well as our named executive officers individual performance factors, have not yet been determined for 2009.
We believe that our annual cash incentive bonus plans help to attract and motivate our executives, and to align the compensation payable to our executives with our corporate objectives, thereby maximizing shareholder value. By evaluating our bonus program for executives each fiscal year, we believe we provide sufficient and attainable incentives for our executives that align with both our financial and non-financial goals.
Equity Incentive Compensation
We believe that our long-term performance is best facilitated through a culture of executive ownership that encourages long-term investment by our executive officers in our equity, thereby better aligning the executives interests with the interests of our stockholders. To encourage this ownership culture, we typically make an initial equity award of stock options to new employees and periodic grants at other times, as approved by our board of directors. Our compensation committee recommends and our board of directors approves all equity grants to our employees including our executive officers. These grants have an exercise price that is at least equal to the fair market value of our common stock on the date of grant, as determined by our board of directors. Grants of options in 2009 were typically subject to a four-year vesting schedule with 1/4th of the grant vesting upon the first anniversary of the vesting commencement date and the remainder of the shares vesting at a rate of 1/48th of the total shares subject to the option each month after the vesting commencement date, subject to the continued service of the executive officer. Vesting commencement dates generally correlate to the date of hire, date of promotion or date of grant. In keeping with our market-competitive philosophy, our compensation committee established the foregoing vesting schedules for 2009 because it determined such vesting represents market practice in our industry based on the experience of the members of our compensation committee.
The size of the initial stock option award is determined based on the executives position with us and takes into account the executives base salary and other compensation as well as an analysis of the grant and compensation practices of the peer companies that we review in connection with establishing our overall compensation policies. The initial stock option awards are intended to provide the executive with an incentive to build value in the organization over an extended period of time while remaining consistent with our overall compensation philosophy.
In 2009, we considered a number of factors in determining the amount of periodic equity incentive awards, if any, granted to our executives, including:
| | the number of shares subject to outstanding options, both vested and unvested, held by our executives; |
| | the vesting schedule of the unvested stock options held by our executives; and |
| | the periodic equity incentive award practices observed in the surveys we reviewed. |
In February 2009, our compensation committee determined that in order to best serve our retention goals, all 2009 refresher stock option grants would vest and become exercisable according to the following schedule: no shares vest until the 24th month following the vesting commencement date, after which 1/24th of the number of shares subject to the grant vest each month. Our named executive officers received the following refresher stock option grants in June 2009, each having an exercise price of $4.97 per share: Dr. Shaw (400,000 shares), Mr. Breuil (100,000 shares), Dr. Anton (35,000 shares), Dr. Sarret (20,000 shares) and Mr. Sheehy (50,000 shares). The size of grant was based on the compensation committees review of data from surveys we considered, grants made to individuals at similar levels within the Company and correlated with the level of authority and responsibility of the named executive officer. Similar to our initial stock option
115
Table of Contents
grants, these refresher grants are intended to continue to provide the executive with an incentive to build value in the organization over an extended period of time while remaining consistent with our overall compensation philosophy. In addition to his refresher grant, Dr. Anton received stock options to purchase 35,000 shares and 50,000 shares of our common stock for an exercise price of $4.97 per share in June 2009, which our board of directors determined was the per share fair market value of our common stock as of the date of grant, in connection with promotions in February and May 2009. He currently serves as our Senior Vice President, Research and Development. In addition to his refresher grant, Dr. Sarret received stock options to purchase 55,500 shares and 180,000 shares of our common stock for exercise prices of $4.97 per share and $6.06 per share, respectively, in June and November 2009, which our board of directors determined was the per share fair market value of our common stock as of the date of grant, in connection with promotions in February and October 2009. He currently serves as our Chief Business Officer and President, Pharmaceutical Services and Enzyme Products. Additionally, Mr. Sheehy received a stock option to purchase 61,000 shares of our common stock for an exercise price of $6.06 per share in November 2009, which our board of directors determined was the per share fair market value of our common stock as of the date of grant, in connection with his promotion to Senior Vice President, General Counsel and Secretary. The size of Dr. Sarrets, Dr. Antons and Mr. Sheehys grants was determined based on the relative size of option grants provided to other executive officers.
Mr. Lawson was granted an initial stock option to purchase 400,000 shares of our common stock for an exercise price of $6.06 per share, which our board of directors determined was the per share fair market value of our common stock as of the date of grant, in connection with his commencement of employment with our company in November 2009. This award vests and becomes exercisable according to the following schedule: 1/4th of the shares vest on the one year anniversary of the commencement of his employment with us and the remainder of the shares vest at a rate of 1/48th of the total shares subject to the option each month thereafter, subject to his continued service.
As a privately owned company, there has been no market for our common stock. Accordingly, in 2009, we had no program, plan or practice pertaining to the timing of stock option grants to executive officers coinciding with the release of material non-public information. The compensation committee intends to adopt a formal policy regarding the timing of grants in connection with this offering.
Termination-Based Compensation
Our compensation committee provides our executives with termination protection when it determines that such protection is necessary to attract or retain an executive.
We have entered into change in control agreements with Dr. Shaw, Mr. Breuil, Dr. Sarret, Mr. Lawson and Mr. Sheehy, which provide severance payments and benefits in the event the executive is terminated without cause, resigns with good reason, or terminates for death or disability within 12 months following or, in certain circumstances, when the executive is terminated without cause or resigns with good reason within a short period prior to a change in control of our company, defined generally as our dissolution or liquidation; a sale of all or substantially all of our assets; a merger, acquisition or consolidation in which the beneficial ownership of our securities representing at least 50% of the combined voting power entitled to vote in the election of our directors has changed; or if current members of our board of directors, or their successors if approved by the vote of at least 50% of the current board, cease to constitute at least 50% of our board of directors, each as further set forth in the individual agreements.
The severance payments and benefits that are payable under the change in control agreements are further described below in the section entitled Potential Payments Upon Termination and Change in Control Change in Control Agreements.
116
Table of Contents
Other Compensation
All of our executive officers are eligible to participate in certain benefit plans and arrangements offered to employees generally, including health, dental, life and disability insurance and our 401(k) plan. We currently pay in excess of 85% of the monthly premium, with respect to coverage for the employee only portion of coverage for all employees, including our named executive officers, for medical, dental, vision, life and long-term disability insurance. Should medical insurance premium rates increase, employees, including named executive officers, may be required to contribute to the cost of increased premiums to retain coverage. Consistent with our market-competitive compensation philosophy, we intend to continue to maintain these benefit plans and arrangements for our employees, including our executive officers. Our compensation committee in its discretion may revise, amend or add to any executives benefits and perquisites if it deems it advisable. We currently do not believe it is necessary for the attraction or retention of management talent to provide the officers with a substantial amount of compensation in the form of perquisites.
Tax Considerations
Section 162(m) of the Internal Revenue Code of 1986, as amended, or the Code, generally disallows a tax deduction for compensation in excess of $1.0 million paid to certain named executive officers. Qualifying performance-based compensation is not subject to the deduction limitation if specified requirements are met. We generally intend to structure the performance-based portion of our executive compensation, when feasible, to comply with exemptions in Section 162(m) so that the compensation remains tax deductible to us. However, our board of directors may, in its judgment, authorize compensation payments that do not comply with the exemptions in Section 162(m) when it believes that such payments are appropriate to attract and retain executive talent.
117
Table of Contents
2009 Summary Compensation Table
The following table summarizes the compensation that we paid to our Chief Executive Officer, Chief Financial Officer and each of our three other most highly compensated executive officers during the year ended December 31, 2009. We refer to these officers in this prospectus as our named executive officers.
| Name |
Year |
Salary ($) |
Bonus ($) |
Option Awards ($)(1) |
Non-Equity Incentive Plan Compensation ($)(2) |
All Other Compensation ($) |
Total ($) |
|||||||||||||||
| Alan Shaw, Ph.D., President and Chief Executive Officer |
2009 2008 2007 |
$ |
425,000 425,000 385,000 |
$ |
149,899 |
|
$ |
1,368,640 1,472,329 |
$ |
|
$
|
|
|
$ |
1,793,640 574,899 2,117,204 |
|||||||
| Robert J. Lawson, Senior Vice President and Chief Financial Officer(3) |
2009 | 55,000 | 50,000 | (4) | 1,602,640 | | 149 | (5) | 1,707,789 | |||||||||||||
| Douglas T. Sheehy, Senior Vice President, General Counsel and Secretary |
2009 2008 2007 |
|
272,660 260,000 164,522 |
|
55,022 |
|
|
415,483 313,604 |
|
|
|
|
|
|
688,143 315,022 557,326 |
|||||||
| David L. Anton, Ph.D., Senior Vice President, Research & Development |
2009 2008 |
|
260,308 176,250 |
|
42,019 |
|
|
403,265 671,640 |
|
|
|
1,110 146,583 |
(6)
|
|
664,683 1,036,492 |
|||||||
| Joseph J. Sarret, M.D., J.D., Chief Business Officer and President, Pharmaceutical Services and Enzyme Products |
2009 | 275,417 | | 974,735 | | 6,309 | (7) | 1,256,461 | ||||||||||||||
| Robert S. Breuil, Former Senior Vice President, Finance and Chief Financial Officer(8) |
2009 2008 2007 |
|
160,000 320,000 288,750 |
|
72,234 |
|
|
342,160 577,315 |
|
|
|
194,976 |
(9)
|
|
697,136 392,234 999,973 |
|||||||
| (1) | The amounts included in the Option Awards column represent the grant date fair value calculated in accordance with ASC Topic 718. The valuation assumptions used in determining such amounts are described in Note 12 to our consolidated financial statements included in this prospectus. |
| (2) | Amounts reflect bonus payments made pursuant to the applicable executive incentive compensation plan. The bonus amount for each named executive officer in 2009, with the exception of Mr. Lawson, under the 2009 Executive Incentive Bonus Plan is zero since the final bonus amounts have not yet been determined. Please refer to the Grants of Plan-Based Awards in 2009 Table for the expected range of bonus payouts for each of our named executive officers. Mr. Lawson was not eligible for the executive incentive compensation plan in 2009. |
| (3) | Mr. Lawson joined Codexis as Senior Vice President and Chief Financial Officer in November 2009. |
| (4) | Represents amount paid as new hire bonus of $50,000. |
| (5) | Represents long-term disability insurance premiums. |
| (6) | Represents long-term disability insurance premiums of $703 and amount paid to reimburse health club membership of $407. |
| (7) | Represents additional medical benefits of $5,413 and long-term disability premiums of $896. |
| (8) | Mr. Breuils employment with us terminated effective as of June 30, 2009. |
| (9) | Represents severance pay amounting to $160,000, paid vacation and time-off of $34,257 and long-term disability premiums of $719. |
118
Table of Contents
Grants of Plan-Based Awards in 2009 Table
All options granted to our named executive officers are incentive stock options, to the extent permissible under the Code. The exercise price per share of each option granted to our named executive officers was determined to be equal to at least the fair market value of our common stock by our board of directors on the date of the grant. All options were granted under our 2002 Stock Plan, as amended, as described below in the section entitled Employee Benefit and Stock Plans 2002 Stock Plan, as amended.
The following table shows information regarding grants of equity awards during the year ended December 31, 2009 to each of our named executive officers.
| Name |
Grant Date |
Estimated Future Payouts Under Non-Equity Incentive Plan Awards($)(1) |
All Other Option Awards; Number of Securities Underlying Options (#) |
Exercise or Base Price of Option Awards ($/Share) |
Grant Date Fair Value of Option Awards ($)(2) |
||||||||||||||
| Threshold | Target | Maximum | |||||||||||||||||
| Alan Shaw, Ph.D. |
| $ | 136,000 | $ | 212,500 | $ | 382,500 | | $ | | $ | | |||||||
| 6/2/2009 | | | | 400,000 | 4.97 | 3.42 | |||||||||||||
| Robert J. Lawson(3) |
11/9/2009 | | | | 400,000 | 6.06 | 4.01 | ||||||||||||
| Douglas T. Sheehy |
| 55,154 | 86,178 | 155,121 | | | | ||||||||||||
| 6/2/2009 | | | | 50,000 | 4.97 | 3.42 | |||||||||||||
| 11/9/2009 | | | | 61,000 | 6.06 | 4.01 | |||||||||||||
| David L. Anton, Ph.D. |
| 59,859 | 93,529 | 168,353 | | | | ||||||||||||
| 6/2/2009 | | | | 35,000 | 4.97 | 3.34 | |||||||||||||
| 6/2/2009 | | | | 50,000 | 4.97 | 3.34 | |||||||||||||
| 6/2/2009 | | | | 35,000 | 4.97 | 3.42 | |||||||||||||
| Joseph J. Sarret, M.D., J.D. |
| 68,096 | 106,400 | 191,520 | | | | ||||||||||||
| 6/2/2009 | | | | 55,500 | 4.97 | 3.34 | |||||||||||||
| 6/2/2009 | | | | 20,000 | 4.97 | 3.42 | |||||||||||||
| 11/9/2009 | | | | 180,000 | 6.06 | 4.01 | |||||||||||||
| Robert S. Breuil |
6/2/2009 | | | | 100,000 | 4.97 | 3.42 | ||||||||||||
| (1) | Amounts in the Estimated Future Payouts Under Non-Equity Incentive Plan Awards column relate to amounts payable under our Executive Incentive Compensation Plan. The threshold column assumes the achievement of either the corporate or individual goals at the threshold level. The maximum column assumes the maximum achievement for both corporate and individual goals. |
| (2) | The amount set forth in the Grant Date Fair Value of Option Awards column are the per share full grant date fair value of the award determined in accordance with ASC Topic 718. The valuation assumptions used in determining such amounts are described in Note 12 to our consolidated financial statements included in this prospectus. |
| (3) | Employees whose date of hire is after October 1, 2009 are not be eligible for a bonus payout from the 2009 Executive Incentive Compensation Plan. Mr. Lawson joined Codexis in November 2009 and, therefore, he is not eligible to participate in the 2009 Executive Incentive Compensation Plan. |
119
Table of Contents
Outstanding Equity Awards at 2009 Fiscal Year-End
The following table shows grants of stock options outstanding on December 31, 2009, the last day of our fiscal year, to each of our named executive officers.
| Option Awards | ||||||||||||
| Name |
Vesting Commencement Date |
Number of Securities Underlying Unexercised Options (#) Exercisable(1) |
Number of Securities Underlying Unexercised Options (#) Unexercisable(1) |
Option Exercise Price ($) |
Option Expiration Date |
|||||||
| Alan Shaw, Ph.D. |
5/16/2003 | (2) | 500,000 | 0 | $ | 0.40 | 5/16/2013 | |||||
| 7/15/2003 | (3) | 50,000 | 0 | 0.40 | 7/15/2013 | |||||||
| 1/1/2004 | 140,000 | 0 | 0.40 | 12/11/2013 | ||||||||
| 1/1/2005 | 80,000 | 0 | 0.60 | 1/5/2015 | ||||||||
| 1/1/2005 | (4) | 20,000 | 0 | 0.60 | 1/5/2015 | |||||||
| 10/18/2005 | 50,000 | 0 | 0.70 | 10/18/2015 | ||||||||
| 1/1/2006 | (4) | 70,000 | 0 | 0.70 | 12/13/2015 | |||||||
| 8/23/2006 | 180,937 | 36,188 | 1.63 | 1/26/2017 | ||||||||
| 12/31/2006 | 162,843 | 54,282 | 1.63 | 1/26/2017 | ||||||||
| 8/28/2007 | 196,874 | 140,626 | 4.47 | 8/28/2017 | ||||||||
| 10/25/2007 | 94,249 | 79,751 | 4.57 | 10/25/2017 | ||||||||
| 1/1/2009 | (6) | 0 | 400,000 | 4.97 | 6/2/2019 | |||||||
| Robert J. Lawson |
11/2/2009 | 0 | 400,000 | 6.06 | 11/9/2019 | |||||||
| Douglas T. Sheehy |
4/2/2007 | 99,999 | 50,001 | 1.63 | 4/19/2017 | |||||||
| 8/28/2007 | 19,249 | 13,751 | 4.47 | 8/28/2017 | ||||||||
| 10/25/2007 | 30,333 | 25,667 | 4.57 | 10/25/2017 | ||||||||
| 1/1/2009 | (6) | 0 | 50,000 | 4.97 | 6/2/2019 | |||||||
| 11/9/2009 | 0 | 61,000 | 6.06 | 11/9/2019 | ||||||||
| David L. Anton, Ph.D. |
3/24/2008 | 65,624 | 84,376 | 7.90 | 5/22/2018 | |||||||
| 1/1/2009 | (6) | 0 | 35,000 | 4.97 | 6/2/2019 | |||||||
| 3/1/2009 | 0 | 35,000 | 4.97 | 6/2/2019 | ||||||||
| 5/12/2009 | 0 | 50,000 | 4.97 | 6/2/2019 | ||||||||
| Joseph J. Sarret, M.D., J.D. |
8/1/2005 | 55,000 | 0 | 0.70 | 8/11/2015 | |||||||
| 1/26/2007 | 58,333 | 21,667 | 1.63 | 1/26/2017 | ||||||||
| 8/28/2007 | 17,208 | 12,292 | 4.47 | 8/28/2017 | ||||||||
| 10/25/2007 | 32,499 | 27,501 | 4.57 | 10/25/2017 | ||||||||
| 1/1/2009 | (6) | 0 | 20,000 | 4.97 | 6/2/2019 | |||||||
| 3/1/2009 | 0 | 55,500 | 4.97 | 6/2/2019 | ||||||||
| 10/16/2009 | 0 | 180,000 | 6.06 | 11/9/2019 | ||||||||
| Robert S. Breuil(5) |
1/3/2006 | 256,249 | 0 | 0.70 | 6/30/2012 | |||||||
| 1/3/2006 | 53,171 | 0 | 1.63 | 6/30/2012 | ||||||||
| 12/31/2006 | 38,906 | 0 | 1.63 | 6/30/2012 | ||||||||
| 8/28/2007 | 49,499 | 0 | 4.47 | 6/30/2012 | ||||||||
| 10/25/2007 | 44,999 | 0 | 4.57 | 6/30/2012 | ||||||||
| (1) | Unless otherwise noted, each option vests as to 1/4th of the total number of shares subject to the option on the first anniversary of the vesting commencement date, and 1/48th of the total number of shares subject to the option shall vest monthly thereafter until all shares are vested. |
120
Table of Contents
| (2) | These options vest as to 1/4th of the total number of shares subject to the option on the six month anniversary of the vesting commencement date, and 1/48th of the total number of shares subject to the option shall vest monthly thereafter. |
| (3) | These options vest as to 100% of the total number of shares subject to the option on the fifth anniversary of the vesting commencement date. |
| (4) | These options were fully vested on the date of grant. |
| (5) | Mr. Breuil will be able to exercise his vested stock options until the earliest of (a) June 30, 2012, (b) the closing of a change in control (as defined in the Plan) of our company or (c) the later of (A) the six month anniversary of expiration of any lock-up or similar transfer restriction imposed on the shares of any common stock underlying his stock options in connection with this offering and (B) the twelve month anniversary of this offering. Effective June 30, 2009, all of Mr. Breuils unvested options to purchase 298,973 shares of our common stock were terminated. |
| (6) | These options vest according to the following schedule: no shares vest until the 24th month following the vesting commencement date, after which 1/24th of the number of shares subject to the grant vest each month. |
Option Exercises and Stock Vested in 2009
None of our named executive officers exercised stock options during 2009 and none of our named executive officers hold stock awards.
Pension Benefits
We do not maintain any defined benefit pension plans.
Nonqualified Deferred Compensation
We do not maintain any nonqualified deferred compensation plans.
Offer Letter Agreements
We have entered into the following offer letter agreements with our named executive officers.
Alan Shaw, Ph.D. On July 29, 2003, we entered into an offer letter agreement with Dr. Shaw, setting forth the terms and conditions of his employment as our Chief Executive Officer. The offer letter agreement provided for annual base salary of $285,000. The offer letter agreement also provided that for 2003, Dr. Shaw would be eligible to participate in our Executive Bonus Plan for 2003, a performance-based program that allowed for a bonus stock option award based upon achievement of our objectives. In connection with his offer letter agreement, Dr. Shaw was granted an option to purchase shares of common stock of our company in exchange for cancellation of his options to purchase shares of Maxygen, Inc.
Robert J. Lawson. On October 16, 2009, we entered into an offer letter agreement with Mr. Lawson, setting forth the terms and conditions of his employment as our Senior Vice President and Chief Financial Officer. The offer letter agreement provided an annual base salary of $330,000. The offer letter agreement also provided that he is eligible to participate in our Executive Incentive Compensation Plan starting in fiscal year 2010, with a target of 40% of his annualized base salary, and which will be awarded based on the companys, as well as Mr. Lawsons individual, performance. Mr. Lawson also was eligible to receive a sign-on bonus of $50,000, which was contingent upon his starting work with the company on or prior to November 2, 2009. Mr. Lawson will be required to pay back the bonus if he chooses to resign with one year in the following amounts: (i) in full if he resigns within three months of his date of hire, and (ii) prorated monthly if he resigns between three and twelve months after commencing employment. In connection with Mr. Lawsons commencement of employment, he received an option to purchase 400,000
121
Table of Contents
shares of our common stock for an exercise price per share equal to $6.06, which option vests as to 1/4th of the total number of shares subject to the option on the first anniversary of his commencement of employment, and 1/48th of the total number of shares subject to the option vesting monthly thereafter until all shares are vested. The offer letter also provided that Mr. Lawson would enter into a Change in Control Agreement upon his commencement of employment with our company.
Douglas T. Sheehy. On February 26, 2007, we entered into an offer letter agreement with Mr. Sheehy, setting forth the terms and conditions of his employment as our Vice President, General Counsel and Secretary. The offer letter agreement provided an annual base salary of $220,000. The offer letter also provided that he is eligible to participate in our Executive Incentive Compensation Plan, with a target of 30% of his annualized base salary (prorated to his start date) for 2007, and which will be awarded at the discretion of our board of directors based on the companys performance. Mr. Sheehy also was eligible to receive a signing bonus of up to $40,000, which was to be offset by any 2006 year-end bonus that he received from his previous employer. Because Mr. Sheehy received his full year-end bonus from his previous employer, he did not receive any signing bonus from us. In connection with the offer letter agreement, Mr. Sheehy received an option to purchase 150,000 shares of our common stock for an exercise price per share equal to $1.63, which option vests as to 1/4th of the total number of shares subject to the option on the first anniversary of the vesting commencement date, and 1/48th of the total number of shares subject to the option vesting monthly thereafter until all shares are vested. The offer letter also provided that at the time of the company wide compensation review following December 31, 2007, Mr. Sheehy would receive an option to purchase a minimum of 33,000 shares of our common stock, contingent upon Mr. Sheehys performance and subject to the approval of our board of directors. In lieu of this option grant, Mr. Sheehy received options to purchase 33,000 and 56,000 shares of our common stock on August 28, 2007 and October 25, 2007, respectively. The offer letter provides for certain benefits payable to Mr. Sheehy in the event of termination following a change in control of our company, as described below in the section entitled Potential Payments Upon Termination and Change in Control Change in Control Agreements.
David L. Anton, Ph.D. On February 15, 2008, we entered into an offer letter agreement with Dr. Anton, setting forth the terms and conditions of his employment as our Vice President, Bioindustrial Research and Development. The offer letter agreement provided an annual base salary of $235,000. The offer letter agreement also provided that he is eligible to participate in our Executive Incentive Compensation Plan, with a target of 25% of his annualized base salary (prorated to his start date) for 2008, and which will be awarded at the discretion of our board of directors based on the companys performance. Dr. Anton also was eligible to receive a signing bonus of up to $10,000, which was contingent upon his starting work with the company on or prior to March 24, 2008. In connection with Dr. Antons commencement of employment, he received an option to purchase 150,000 shares of our common stock for an exercise price per share equal to $7.90, which option vests as to 1/4th of the total number of shares subject to the option on the first anniversary of the vesting commencement date, and 1/48th of the total number of shares subject to the option vesting monthly thereafter until all shares are vested. The offer letter also provided for relocation assistance in an amount to be determined at a later date. When paid, Dr. Anton received a total of $146,582 in relocation assistance.
Joseph J. Sarret, M.D., J.D. On January 25, 2007, we entered into an offer letter agreement with Dr. Sarret, setting forth the terms and conditions of his promotion to Vice President, Corporate Development, which superseded all prior agreements relating to his employment with our company. The offer letter agreement provided an annual base salary of $190,000. The offer letter agreement also provided that he is eligible for a performance-based discretionary cash bonus, prorated to his promotion date, with a target of 20% of his annualized base salary, and which will be awarded based on our corporate, as well as Dr. Sarrets individual, performance. In connection with Dr. Sarrets promotion, he received an option to purchase 80,000 shares of our common stock for an exercise price per share equal to $1.63, which option vests as to 1/4th of the total number of shares subject to the option on the first anniversary of his promotion, and 1/48th of the total number of shares subject to the option vesting monthly thereafter until
122
Table of Contents
all shares are vested. Dr. Sarrets offer letter further provides that his original stock option grant having a vesting commencement date of August 1, 2005, shall continue to vest in accordance with the vesting schedule in the original grant.
Robert S. Breuil. On December 22, 2005, we entered into an offer letter agreement with Mr. Breuil, setting forth the terms and conditions of his employment as our Senior Vice President, Finance and Chief Financial Officer. The offer letter agreement provided for annual base salary of $275,000. Mr. Breuils offer letter agreement provided that for 2006, he would be eligible to participate in an Executive Bonus Plan, and that the bonus would be paid out in the form of stock options or cash, or a combination of cash and stock options at the discretion of our compensation committee, based upon the achievement of corporate and individual objectives as defined by our Chief Executive Officer and our board of directors, and subject to the final approval of our compensation committee. The offer letter agreement provided that the dollar value of the bonus payout for the Senior Vice President level is 30% of annual base salary.
In connection with the offer letter agreement, Mr. Breuil received an option to purchase 300,000 shares of our common stock for an exercise price per share equal to $0.70, which option vests as to 1/4th of the total number of shares subject to the option on the first anniversary of his employment start date, and 1/48th of the total number of shares subject to the option vesting monthly thereafter until his employment with us is terminated. In addition, the offer letter provided for an additional grant of an option to purchase shares, following the closing of our companys next financing following the date of the offer letter agreement, in a total share amount equal to the amount necessary to make Mr. Breuils then-total ownership of our company equal to 1.25% of our then fully diluted shares (the Supplemental Hire Grant). The offer letter provided that Mr. Breuil would be eligible for periodic stock option grants based upon our companys and his individual performance, with his target total stock and option ownership, including vested and unvested shares, but excluding any option shares granted pursuant to our Executive Bonus Plan, expected to be approximately 1.25% of our fully diluted shares outstanding immediately prior to our filing to complete an initial public offering.
On June 30, 2009, we entered into a Separation Agreement with Mr. Breuil in connection with his resignation of employment with us. Pursuant to the Separation Agreement, in return for a full release of claims against us and our affiliates, we provided Mr. Breuil cash lump sum severance in the amount of $160,000 and reimbursed him for six months of COBRA coverage for him and his dependents. The post-termination exercise period with respect to Mr. Breuils vested options was also extended to the earliest of (i) the third anniversary of his termination of employment, (ii) the closing of a change in control and (iii) the later of the 12-month anniversary of this offering or the six-month anniversary of the expiration of any lock-up restriction imposed on Mr. Breuil in connection with this offering. Mr. Breuil also agreed to be available to consult with us on a paid and as-needed basis for three months following his termination of employment, and has continued to consult for us beyond that three-month period.
Change in Control Agreements
During 2009, we were party to change in control agreements with Dr. Shaw, Mr. Breuil, Dr. Sarret, Mr. Lawson and Mr. Sheehy. The change in control agreements provide that in the event a named executive officer is terminated without cause or resigns for good reason, each as defined in the agreements, within twelve months following the change in control of our company, the terminated executive officer is entitled, subject to our receipt of a release of claims and a confidential information, secrecy and invention agreement, to the following payments and benefits:
| Base salary, payable in a cash lump sum |
12 months | |
| Equity award vesting acceleration |
100% | |
| Continued healthcare coverage premiums(1) |
12 months |
| (1) | If an executive elects to receive continued healthcare coverage pursuant to the provisions of COBRA, the executive will be eligible for reimbursement or direct payment of COBRA coverage premiums for the |
123
Table of Contents
| executive and any dependents. If the executive and/or the executives dependents become eligible for healthcare coverage under a subsequent employers plans, payment of coverage premiums will cease. |
The following table sets forth quantitative estimates of the benefits that would have accrued to each of our named executive officers if his employment had been terminated on December 31, 2009 by us without cause or for good reason by the named executive officers upon a change in control, assuming that such termination occurred within the period beginning on the effective date of a change in control as specified in the agreement and ending on the last day of the twelfth calendar month following the calendar month in which the effective date of a change in control occurs. Amounts below reflect potential payments pursuant to the change in control agreements for such named executive officers.
| Name |
Salary Continuation |
Value of Accelerated Equity Awards(1) |
Value of Continued Healthcare Coverage |
Total | ||||||||
| Alan Shaw, Ph.D. |
$ | 425,000 | $ | 1,826,077 | $ | 24,347 | $ | 2,275,424 | ||||
| Robert J. Lawson |
330,000 | 364,000 | 24,347 | 718,347 | ||||||||
| Douglas T. Sheehy |
300,000 | 518,494 | 24,347 | 842,841 | ||||||||
| Joseph J. Sarret, M.D., J.D. |
320,000 | 527,234 | 13,164 | 860,398 | ||||||||
| (1) | Amounts calculated based on the aggregate amount by which the fair market value of the common stock subject to unvested equity awards exceeded the aggregate exercise price of the awards as of December 31, 2009. |
In addition, during 2009 Dr. Shaw, Mr. Lawson, Dr. Sarret, Mr. Breuil and Mr. Sheehy were entitled to equity award vesting acceleration with respect to that number of shares that would otherwise have vested through the next vesting date following the executives termination, pro-rated to the date of termination, and continued healthcare coverage premiums for one year from the date of termination, in the event they were terminated for death or disability, both as defined in their respective agreements, within twelve months following a change of control of our company. The value of the accelerated equity awards as of December 31, 2009 were as follows: Dr. Shaw ($25,556), Mr. Lawson ($14,709), Mr. Sheehy ($20,280) and Dr. Sarret ($34,663). Mr. Breuil terminated his employment with the company in June 2009. The value of the continued healthcare coverage premium can be found in the preceding table in the Value of Continued Healthcare Coverage column.
Confidentiality Information, Secrecy and Invention Agreements
Each of our named executive officers has entered into a standard form agreement with respect to confidential information, secrecy and inventions. Among other things, this agreement obligates each named executive officer to refrain from disclosing any of our proprietary information received during the course of employment and, with some exceptions, to assign to us any inventions conceived or developed during the course of employment.
Employee Benefit and Stock Plans
2010 Equity Incentive Award Plan
We intend to adopt a 2010 Equity Incentive Award Plan, or the 2010 Plan, which will be effective on the date of adoption. The principal purpose of the 2010 Plan is to attract, retain and motivate selected employees, consultants and directors through the granting of stock-based compensation awards and cash-based performance bonus awards. The 2010 Plan is also designed to permit us to make cash-based awards and equity-based awards intended to qualify as performance-based compensation under Section 162(m) of the Internal Revenue Code of 1986, as amended, or the Code.
The principal features of the 2010 Plan are summarized below. This summary is qualified in its entirety by reference to the text of the 2010 Plan, which is filed as an exhibit to the registration statement of which this prospectus is a part.
124
Table of Contents
Share Reserve. Under the 2010 Plan, shares of our common stock will be initially reserved for issuance pursuant to a variety of stock-based compensation awards, including stock options, stock appreciation rights, or SARs, restricted stock awards, restricted stock unit awards, deferred stock awards, dividend equivalent awards, stock payment awards and performance awards and other stock-based awards, plus the number of shares remaining available for future awards under our 2002 Stock Plan as of the completion of this offering. The number of shares initially reserved for issuance or transfer pursuant to awards under the 2010 Plan will be increased by (i) the number of shares represented by awards outstanding under our 2002 Stock Plan that are forfeited or lapse unexercised and which following the effective date are not issued under the 2002 Stock Plan and (ii) an annual increase on the first day of each fiscal year beginning in 2011 and ending in 2020, equal to the least of (A) shares, (B) % of the shares of stock outstanding (on an as converted basis) on the last day of the immediately preceding fiscal year and (C) such smaller number of shares of stock as determined by our board of directors; provided, however, no more than shares of stock may be issued upon the exercise of incentive stock options.
The following counting provisions will be in effect for the share reserve under the 2010 Plan:
| | to the extent that an award terminates, expires or lapses for any reason or an award is settled in cash without the delivery of shares, any shares subject to the award at such time will be available for future grants under the 2010 Plan; |
| | to the extent shares are tendered or withheld to satisfy the grant, exercise price or tax withholding obligation with respect to any award under the 2010 Plan, such tendered or withheld shares will be available for future grants under the 2010 Plan; |
| | to the extent that shares of our common stock are repurchased by us prior to vesting so that shares are returned to us, such shares will be available for future grants under the 2010 Plan; |
| | the payment of dividend equivalents in cash in conjunction with any outstanding awards will not be counted against the shares available for issuance under the 2010 Plan; and |
| | to the extent permitted by applicable law or any exchange rule, shares issued in assumption of, or |
| | in substitution for, any outstanding awards of any entity acquired in any form of combination by us or any of our subsidiaries will not be counted against the shares available for issuance under the 2010 Plan. |
Administration. The compensation committee of our board of directors will administer the 2010 Plan unless our board of directors assumes authority for administration. The compensation committee must consist of at least two members of our board of directors, each of whom is intended to qualify as an outside director, within the meaning of Section 162(m) of the Code, a non-employee director for purposes of Rule 16b-3 under the Securities Exchange Act of 1934, as amended, or the Exchange Act, and an independent director within the meaning of the rules of The Nasdaq Stock Market, or other principal securities market on which shares of our common stock are traded. The 2010 Plan provides that the compensation committee may delegate its authority to grant awards to employees other than executive officers and certain senior executives of the company to a committee consisting of one or more members of our board of directors or one or more of our officers, but our compensation committee charter prohibits such delegation in the case of awards to employees at or above the level of vice president, and the equity awards policy we adopted in , 2010 calls for the compensation committee to approve all equity awards, other than awards made to our non-employee directors, which must be approved by our full board of directors.
Subject to the terms and conditions of the 2010 Plan, the administrator has the authority to select the persons to whom awards are to be made, to determine the number of shares to be subject to awards and the terms and conditions of awards, and to make all other determinations and to take all other actions necessary or advisable for the administration of the 2010 Plan. The administrator is also authorized to adopt, amend
125
Table of Contents
or rescind rules relating to administration of the 2010 Plan. Our board of directors may at any time remove the compensation committee as the administrator and revest in itself the authority to administer the 2010 Plan. The full board of directors will administer the 2010 Plan with respect to awards to non-employee directors.
Eligibility. Options, SARs, restricted stock and all other stock-based and cash-based awards under the 2010 Plan may be granted to individuals who are then our officers, employees or consultants or are the officers, employees or consultants of certain of our subsidiaries. Such awards also may be granted to our directors. Only employees of our company or certain of our subsidiaries may be granted incentive stock options, or ISOs.
Awards. The 2010 Plan provides that the administrator may grant or issue stock options, SARs, restricted stock, restricted stock units, deferred stock, dividend equivalents, performance awards, stock payments and other stock-based and cash-based awards, or any combination thereof. Each award will be set forth in a separate agreement with the person receiving the award and will indicate the type, terms and conditions of the award.
| | Nonqualified Stock Options, or NQSOs, will provide for the right to purchase shares of our common stock at a specified price which may not be less than fair market value on the date of grant, and usually will become exercisable (at the discretion of the administrator) in one or more installments after the grant date, subject to the participants continued employment or service with us and/or subject to the satisfaction of corporate performance targets and individual performance targets established by the administrator. NQSOs may be granted for any term specified by the administrator that does not exceed ten years. |
| | Incentive Stock Options, or ISOs, will be designed in a manner intended to comply with the provisions of Section 422 of the Code and will be subject to specified restrictions contained in the Code. Among such restrictions, ISOs must have an exercise price of not less than the fair market value of a share of common stock on the date of grant, may only be granted to employees, and must not be exercisable after a period of ten years measured from the date of grant. In the case of an ISO granted to an individual who owns (or is deemed to own) at least 10% of the total combined voting power of all classes of our capital stock, the 2010 Plan provides that the exercise price must be at least 110% of the fair market value of a share of common stock on the date of grant and the ISO must not be exercisable after a period of five years measured from the date of grant. |
| | Restricted Stock may be granted to any eligible individual and made subject to such restrictions as may be determined by the administrator. Restricted stock, typically, may be forfeited for no consideration or repurchased by us at the original purchase price if the conditions or restrictions on vesting are not met. In general, restricted stock may not be sold or otherwise transferred until restrictions are removed or expire. Purchasers of restricted stock, unlike recipients of options, will have voting rights and will have the right to receive dividends, if any, prior to the time when the restrictions lapse, however, extraordinary dividends will generally be placed in escrow, and will not be released until restrictions are removed or expire. |
| | Restricted Stock Units may be awarded to any eligible individual, typically without payment of consideration, but subject to vesting conditions based on continued employment or service or on performance criteria established by the administrator. Like restricted stock, restricted stock units may not be sold, or otherwise transferred or hypothecated, until vesting conditions are removed or expire. Unlike restricted stock, stock underlying restricted stock units will not be issued until the restricted stock units have vested, and recipients of restricted stock units generally will have no voting or dividend rights prior to the time when vesting conditions are satisfied. |
| | Deferred Stock Awards represent the right to receive shares of our common stock on a future date. Deferred stock may not be sold or otherwise hypothecated or transferred until issued. Deferred |
126
Table of Contents
| stock will not be issued until the deferred stock award has vested, and recipients of deferred stock generally will have no voting or dividend rights prior to the time when the vesting conditions are satisfied and the shares are issued. Deferred stock awards generally will be forfeited, and the underlying shares of deferred stock will not be issued, if the applicable vesting conditions and other restrictions are not met. |
| | Stock Appreciation Rights, or SARs, may be granted in connection with stock options or other awards, or separately. SARs granted in connection with stock options or other awards typically will provide for payments to the holder based upon increases in the price of our common stock over a set exercise price. The exercise price of any SAR granted under the 2010 Plan must be at least 100% of the fair market value of a share of our common stock on the date of grant. Except as required by Section 162(m) of the Code with respect to a SAR intended to qualify as performance-based compensation as described in Section 162(m) of the Code, there are no restrictions specified in the 2010 Plan on the exercise of SARs or the amount of gain realizable therefrom, although restrictions may be imposed by the administrator in the SAR agreements. SARs under the 2010 Plan will be settled in cash or shares of our common stock, or in a combination of both, at the election of the administrator. |
| | Dividend Equivalents represent the value of the dividends, if any, per share paid by us, calculated with reference to the number of shares covered by the award. Dividend equivalents may be settled in cash or shares and at such times as determined by the compensation committee or board of directors, as applicable. |
| | Performance Awards may be granted by the administrator on an individual or group basis. Generally, these awards will be based upon specific performance targets and may be paid in cash or in common stock or in a combination of both. Performance awards may include phantom stock awards that provide for payments based upon the value of our common stock. Performance awards may also include bonuses that may be granted by the administrator on an individual or group basis and which may be payable in cash or in common stock or in a combination of both. |
| | Stock Payments may be authorized by the administrator in the form of common stock or an option or other right to purchase common stock as part of a deferred compensation on other arrangement in lieu of all or any part of compensation, including bonuses, that would otherwise be payable in cash to the employee, consultant or non-employee director. |
Change in Control. In the event of a change in control where the acquiror does not assume or replace awards granted, prior to the consummation of such transaction and then the awards will terminate upon consummation of the transaction under the 2010 Plan, awards issued under the 2010 Plan will be subject to accelerated vesting such that 100% of such awards will become vested and exercisable or payable, as applicable. In addition, the administrator will also have complete discretion to structure one or more awards under the 2010 Plan to provide that such awards will become vested and exercisable or payable on an accelerated basis in the event such awards are assumed or replaced with equivalent awards but the individuals service with us or the acquiring entity is subsequently terminated within a designated period following the change in control event. The administrator may also make appropriate adjustments to awards under the 2010 Plan and is authorized to provide for the acceleration, cash-out, termination, assumption, substitution or conversion of such awards in the event of a change in control or certain other unusual or nonrecurring events or transactions. Under the 2010 Plan, a change in control is generally defined as:
| | the transfer or exchange in a single or series of related transactions by our stockholders of more than 50% of our voting stock to a person or group; |
| | a change in the composition of our board of directors over a two-year period such that 50% or more of the members of the board were elected through one or more contested elections; |
127
Table of Contents
| | a merger, consolidation, reorganization or business combination in which we are involved, directly or indirectly, other than a merger, consolidation, reorganization or business combination which results in our outstanding voting securities immediately before the transaction continuing to represent a majority of the voting power of the acquiring companys outstanding voting securities and after which no person or group beneficially owns 50% or more of the outstanding voting securities of the surviving entity immediately after the transaction; |
| | the sale, exchange, or transfer of all or substantially all of our assets; or |
| | stockholder approval of our liquidation or dissolution. |
Adjustments of Awards. In the event of any stock dividend, stock split, combination or exchange of shares, merger, consolidation, spin-off, recapitalization, distribution of our assets to stockholders (other than normal cash dividends) or any other corporate event affecting the number of outstanding shares of our common stock or the share price of our common stock that would require adjustments to the 2010 Plan or any awards under the 2010 Plan in order to prevent the dilution or enlargement of the potential benefits intended to be made available thereunder, the administrator will make appropriate, proportionate adjustments to:
| | the aggregate number and type of shares subject to the 2010 Plan; |
| | the number and kind of shares subject to outstanding awards and terms and conditions of outstanding awards (including, without limitation, any applicable performance targets or criteria with respect to such awards); and |
| | the grant or exercise price per share of any outstanding awards under the 2010 Plan. |
Amendment and Termination. Our board of directors or the committee (with board approval) may terminate, amend or modify the 2010 Plan at any time and from time to time. However, we must generally obtain stockholder approval:
| | to increase the number of shares available under the 2010 Plan (other than in connection with certain corporate events, as described above); |
| | to grant options with an exercise price that is below 100% of the fair market value of shares of our common stock on the grant date; |
| | to extend the exercise period for an option beyond ten years from the date of grant; or |
| | to the extent required by applicable law, rule or regulation (including any applicable stock exchange rule). |
Notwithstanding the foregoing, an option may be amended to reduce the per share exercise price below the per share exercise price of such option on the grant date and options may be granted in exchange for, or in connection with, the cancellation or surrender of options having a higher per share exercise price without receiving additional shareholder approval.
Expiration Date. The 2010 Plan will expire on, and no option or other award may be granted pursuant to the 2010 Plan after, the tenth anniversary of the effective date of the 2010 Plan. Any award that is outstanding on the expiration date of the 2010 Plan will remain in force according to the terms of the 2010 Plan and the applicable award agreement.
Securities Laws and U.S. Federal Income Taxes. The 2010 Plan is designed to comply with various securities and U.S. federal tax laws as follows:
Securities Laws. The 2010 Plan is intended to conform to all provisions of the Securities Act and the Exchange Act and any and all regulations and rules promulgated by the SEC thereunder, including
128
Table of Contents
without limitation, Rule 16b-3. The 2010 Plan will be administered, and options will be granted and may be exercised, only in such a manner as to conform to such laws, rules and regulations.
Section 409A of the Code. Certain awards under the 2010 Plan may be considered nonqualified deferred compensation for purposes of Section 409A of the Code, which imposes certain additional requirements regarding the payment of deferred compensation. Generally, if at any time during a taxable year a nonqualified deferred compensation plan fails to meet the requirements of Section 409A, or is not operated in accordance with those requirements, all amounts deferred under the 2010 Plan and all other equity incentive plans for the taxable year and all preceding taxable years by any participant with respect to whom the failure relates are includible in gross income for the taxable year to the extent not subject to a substantial risk of forfeiture and not previously included in gross income. If a deferred amount is required to be included in income under Section 409A, the amount also is subject to interest and an additional income tax. The interest imposed is equal to the interest at the underpayment rate plus one percentage point, imposed on the underpayments that would have occurred had the compensation been includible in income for the taxable year when first deferred, or if later, when not subject to a substantial risk of forfeiture. The additional U.S. federal income tax is equal to 20% of the compensation required to be included in gross income. In addition, certain states, including California, have laws similar to Section 409A, which impose additional state penalty taxes on such compensation.
Section 162(m) of the Code. In general, under Section 162(m) of the Code, income tax deductions of publicly held corporations may be limited to the extent total compensation (including, but not limited to, base salary, annual bonus, and income attributable to stock option exercises and other non-qualified benefits) for certain executive officers exceeds $1,000,000 (less the amount of any excess parachute payments as defined in Section 280G of the Code) in any taxable year of the corporation. However, under Section 162(m), the deduction limit does not apply to certain performance-based compensation established by an independent compensation committee that is adequately disclosed to and approved by stockholders. In particular, stock options and SARs will satisfy the performance-based compensation exception if the awards are made by a qualifying compensation committee, the 2010 Plan sets the maximum number of shares that can be granted to any person within a specified period and the compensation is based solely on an increase in the stock price after the grant date. Specifically, the option exercise price must be equal to or greater than the fair market value of the stock subject to the award on the grant date. Under a Section 162(m) transition rule for compensation plans of corporations which are privately held and which become publicly held in an initial public offering, the 2010 Plan will not be subject to Section 162(m) until a specified transition date, which is the earlier of:
| | the material modification of the 2010 Plan; |
| | the issuance of all of the shares of our common stock reserved for issuance under the 2010 Plan; |
| | the expiration of the 2010 Plan; or |
| | the first meeting of our stockholders at which members of our board of directors are to be elected that occurs after the close of the third calendar year following the calendar year in which our initial public offering occurs. |
After the transition date, rights or awards granted under the 2010 Plan, other than options and SARs, will not qualify as performance-based compensation for purposes of Section 162(m) unless such rights or awards are granted or vest upon pre-established objective performance goals, the material terms of which are disclosed to and approved by our stockholders. Thus, after the transition date, we expect that such other rights or awards under the plan will not constitute performance-based compensation for purposes of Section 162(m).
We intend to file with the SEC a registration statement on Form S-8 covering the shares of our common stock issuable under the 2010 Plan.
129
Table of Contents
2002 Stock Plan, as amended
Our board of directors adopted, and our stockholders approved, the 2002 Stock Plan in November 2002. An aggregate of 15,757,642 shares of our common stock is reserved for issuance under the 2002 Stock Plan. The 2002 Stock Plan provides for the grant of ISOs, NQSOs and stock purchase rights. As of September 30, 2009, options to purchase 10,897,854 shares of our common stock at a weighted average exercise price per share of $3.26 remained outstanding under the 2002 Stock Plan. No stock purchase rights have been granted under the 2002 Stock Plan. As of September 30, 2009, options to purchase 3,226,648 shares of our common stock remained available for future issuance pursuant to awards granted under the 2002 Stock Plan.
Our board of directors, or a committee thereof appointed by our board of directors, has the authority to administer the 2002 Stock Plan and the awards granted under it. Following the completion of this offering, no further awards will be granted under the 2002 Stock Plan; all outstanding awards will continue to be governed by their existing terms.
Stock Options. The 2002 Stock Plan provides for the grant of ISOs under the federal tax laws or NQSOs. ISOs may be granted only to employees. NQSOs and stock purchase rights may be granted to employees, directors or consultants. The exercise price of ISOs granted to employees who at the time of grant own stock representing more than 10% of the voting power of all classes of our common stock may not be less than 110% of the fair market value of our common stock on the date of grant, and the exercise price of ISOs granted to any other employees may not be less than 100% of the fair market value of our common stock on the date of grant. The exercise price of NQSOs to employees, directors or consultants who at the time of grant own stock representing more than 10% of the voting power of all classes of our common stock may not be less than 110% of the fair market value of our common stock on the date of grant, and the exercise price of nonstatutory stock options to all other employees, directors or consultants may not be less than 85% of the fair market value of our common stock on the date of grant. Shares subject to options under the 2002 Stock Plan generally vest in a series of installments over an optionees period of service, with a minimum vesting rate of at least 20% per year over five years from the date of grant, except with respect to options granted to officers, directors and consultants. This minimum vesting rate does not apply to recipients of options who are tax residents of Germany.
In general, the maximum term of options granted is ten years. The maximum term of options granted to an optionee who owns stock representing more than 10% of the voting power of all classes of our common stock is five years. If an optionees service relationship with us terminates other than by disability or death, the optionee may exercise the vested portion of any option in such period of time as specified in the optionees option agreement, but in no event will such period be less than 30 days following the termination of service. If an optionees service relationship with us terminates by disability or death, the optionee, or the optionees designated beneficiary, as applicable, may exercise the vested portion of any option in such period of time as specified in the optionees option agreement, but in no event will such period be less than six months following the termination of service. Shares of common stock representing any unvested portion of the option on the date of termination shall immediately cease to be issuable and shall become available for issuance under the 2002 Stock Plan. If, after termination, the optionee does not exercise the option within the time period specified, the option shall terminate and the shares of common stock covered by such option will become available for issuance under the 2002 Stock Plan.
Stock Purchase Rights. The 2002 Stock Plan provides that we may issue stock purchase rights alone, in addition to or in tandem with options granted under the 2002 Stock Plan and/or cash awards made outside of the 2002 Stock Plan. Any stock purchase rights will be governed by a restricted stock purchase agreement. We will have the right to repurchase shares of common stock acquired by the purchaser upon exercise of a stock purchase right upon the termination of the purchasers status as an employee, director or consultant for any reason. The repurchase price for shares acquired by the purchaser upon exercise of a stock purchase right shall be the original price paid by the purchaser. Except with respect to shares
130
Table of Contents
purchased by officers, directors and consultants, the repurchase option shall lapse at a rate of at least 20% per year over five years from the date of purchase; this term does not apply to stock purchase rights granted to individuals who are tax residents of Germany. Once the stock purchase right is exercised, the purchaser shall have rights equivalent to those of our other stockholders.
Corporate Transactions. In the event of a proposed dissolution or liquidation, the administrator of the 2002 Stock Plan has the discretion to take one or more of the following actions: (a) provide that any option or stock purchase right be made exercisable until 10 days prior to such transaction; and (b) provide that the Company repurchase option applicable to any shares purchased upon exercise of an option or stock purchase right shall lapse as to all such shares. To the extent options and stock purchase rights have not been previously exercised, all such options and stock purchase rights will terminate immediately prior to the consummation of the proposed transaction.
In the event of certain corporate transactions, the administrator of the 2002 Stock Plan shall adjust the number of shares of common stock that may be delivered under the 2002 Stock Plan and/or the number class and price of shares of common stock covered by each outstanding option or stock purchase right.
Change in Control. In the event we undergo a change in control, and any surviving corporation does not assume options or stock purchase rights under the 2002 Stock Plan, or substitute an equivalent option of the successor corporation or a parent or subsidiary of the successor corporation, the vesting of options or stock purchase rights held by participants in the 2002 Stock Plan, shall be accelerated and made fully exercisable. The holder of such options or stock purchase rights not assumed or substituted shall be notified by the 2002 Stock Plan administrator that the option or stock purchase right is fully exercisable for a period of 15 days from the date of such notice, and shall be terminated if not exercised within such 15 day period.
Employee Stock Purchase Plan
Our Employee Stock Purchase Plan, which we refer to as our ESPP, was adopted by our board of directors in and approved by our stockholders in . The ESPP is designed to allow our eligible employees and the eligible employees of our participating subsidiaries to purchase shares of common stock, at semi-annual intervals, with their accumulated payroll deductions.
Share Reserve. shares of our common stock are initially reserved for issuance under our ESPP. The number of shares of common stock reserved under our ESPP will automatically increase on the first trading day each year, beginning in 2011, by an amount equal to the least of: (i) percent ( %) of our outstanding shares of common stock outstanding on such date, (ii) shares or (iii) a lesser amount determined by our board of directors. The maximum aggregate number of shares which may be issued over the term of the ESPP is shares. In addition, no participant in our ESPP may be issued or transferred more than $25,000 of shares of common stock pursuant to awards under the ESPP per calendar year.
Offering Periods. The ESPP has a series of successive offering periods, with a new offering period beginning on and each year. Unless otherwise determined by the compensation committee, each offering period has a duration of six months, other than the initial offering period which will begin on the effective date of our initial public offering and will end on .
Eligible Employees. Our employees, and any employees of our subsidiaries that the compensation committee designates as participating in the ESPP, who are scheduled to work more than 20 hours per week for more than five calendar months per year may join an offering period on the start date of that period.
Payroll Deductions. A participant may contribute from 1% to 15% of his or her compensation through payroll deductions, and the accumulated deductions will be applied to the purchase of shares on each semi-annual purchase date. The purchase price per share will be equal to 85% of the fair market value per share of our common stock on the first trading date of an offering period in which a participant is
131
Table of Contents
enrolled or, if lower, 85% of the fair market value per share on the semi-annual purchase date. Semi-annual purchase dates will occur on the last trading day of each offering period. However, not more than shares may be purchased in total by any participant during any offering period. Our compensation committee has the authority to change these limitations for any subsequent offering period.
Change in Control. Should we be acquired by merger or sale of substantially all of our assets or more than 50% of our voting securities, then all outstanding purchase rights may either be assumed by the acquirer or all outstanding purchase rights will be exercised at an early purchase date prior to the effective date of the acquisition. The purchase price in effect for each participant will be equal to 85% of the fair market value per share of our common stock on the first trading date of the offering period in which the participant is enrolled at the time the acquisition occurs or, if lower, 85% of the fair market value per share on the purchase date prior to the acquisition.
Plan Provisions. The ESPP will terminate no later than 10 years after the date our board approved it. The board may at any time amend, suspend or discontinue the ESPP. However, certain amendments may require stockholder approval.
401(k) Plan
In January 2005, we implemented a 401(k) Plan covering certain employees. Currently, all of our U.S.-based employees over the age of 18 are eligible to participate in the 401(k) Plan. Under the 401(k) Plan, eligible employees may elect to reduce their current compensation by up to the lesser of 75% of their base salary and cash compensation or the prescribed annual limit and contribute these amounts to the 401(k) Plan. We may make matching or other contributions to the 401(k) Plan on behalf of eligible employees. In 2009, we did not make any contributions to the 401(k) Plan on behalf of eligible employees. The 401(k) Plan is intended to qualify under Section 401 of the Code so that contributions by employees to the 401(k) Plan, and income earned on the 401(k) Plan contributions, are not taxable to employees until withdrawn from the 401(k) Plan. The trustees under the 401(k) Plan, at the direction of each participant, invest the 401(k) Plan employee salary deferrals in selected investment options.
Limitation on Liability and Indemnification Matters
Our amended and restated certificate of incorporation and amended and restated bylaws, each to be effective upon the completion of this offering, will provide that we will indemnify our directors, officers, employees and agents to the fullest extent permitted by the Delaware General Corporation Law, which prohibits our amended and restated certificate of incorporation from limiting the liability of our directors for the following:
| | any breach of the directors duty of loyalty to us or to our stockholders; |
| | acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law; |
| | unlawful payment of dividends or unlawful stock repurchases or redemptions; and |
| | any transaction from which the director derived an improper personal benefit. |
If Delaware law is amended to authorize corporate action further eliminating or limiting the personal liability of a director, then the liability of our directors will be eliminated or limited to the fullest extent permitted by Delaware law, as so amended. Our amended and restated certificate of incorporation does not eliminate a directors duty of care and, in appropriate circumstances, equitable remedies, such as injunctive or other forms of non-monetary relief, remain available under Delaware law. This provision also does not affect a directors responsibilities under any other laws, such as the federal securities laws or other state or federal laws. Under our amended and restated bylaws, we will also be empowered to enter into
132
Table of Contents
indemnification agreements with our directors, officers, employees and other agents and to purchase insurance on behalf of any person whom we are required or permitted to indemnify.
In addition to the indemnification required in our amended and restated certificate of incorporation and amended and restated bylaws, we have entered into indemnification agreements with each of our directors, and will enter into new indemnification agreements with each of our current directors, officers, and certain employees before the completion of this offering. These agreements provide for the indemnification of our directors, officers, and certain employees for all reasonable expenses and liabilities incurred in connection with any action or proceeding brought against them by reason of the fact that they are or were our agents. We believe that these provisions in our amended and restated certificate of incorporation and amended and restated bylaws and indemnification agreements are necessary to attract and retain qualified persons as directors and officers. Furthermore, we have obtained director and officer liability insurance to cover liabilities our directors and officers may incur in connection with their services to us. This description of the indemnification provisions of our amended and restated certificate of incorporation, our amended and restated bylaws and our indemnification agreements is qualified in its entirety by reference to these documents, each of which is attached as an exhibit to this registration statement.
The limitation of liability and indemnification provisions in our amended and restated certificate of incorporation and amended and restated bylaws may discourage stockholders from bringing a lawsuit against directors for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation against directors and officers, even though an action, if successful, might benefit us and our stockholders. A stockholders investment may be harmed to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act, and is, therefore, unenforceable. There is no pending litigation or proceeding naming any of our directors or officers as to which indemnification is being sought, nor are we aware of any pending or threatened litigation that may result in claims for indemnification by any director or officer.
Rule 10b5-1 Sales Plans
Our directors and executive officers may adopt written plans, known as Rule 10b5-1 plans, in which they will contract with a broker to buy or sell shares of our common stock on a periodic basis. Under a Rule 10b5-1 plan, a broker executes trades pursuant to parameters established by the director or officer when entering into the plan, without further direction from them. The director or officer may amend or terminate the plan in some circumstances. Our directors and executive officers may also buy or sell additional shares outside of a Rule 10b5-1 plan when they are not in possession of material, nonpublic information.
133
Table of Contents
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
We describe below transactions, since our inception, to which we were a party or will be a party, in which:
| | The amounts involved exceeded or will exceed $120,000; and |
| | A director, executive officer, holder of more than 5% of our common stock or any member of their immediate family had or will have a direct or indirect material interest. |
Preferred Stock Issuances
Issuance of Series F Preferred Stock
Between March and November 2009, we sold 5,529,410 shares of Series F preferred stock at a price of $8.50 per share for gross proceeds of approximately $47.0 million. The table below sets forth the number of shares of Series F preferred stock sold to our directors, executive officers and 5% stockholders and their affiliates.
| Name |
Number of Shares of Series F Preferred Stock |
Aggregate Purchase Price |
|||
| Equilon Enterprises LLC dba Shell Oil Products US(1) |
3,529,411 | $ | 30,000,000.00 | ||
| (1) | Chris Streng is one of our directors and is Vice President Finance Manufacturing for Shell Downstream Inc. |
Issuance of Series E Preferred Stock
During November and December 2007, we sold 6,100,305 shares of Series E preferred stock at a price of $8.50 per share for gross proceeds of approximately $51.9 million, and issued an additional 56,470 shares of Series E preferred stock valued at $480,000 to a professional consulting services firm in exchange for their services. The table below sets forth the number of shares of Series E preferred stock sold to our directors, executive officers and 5% stockholders and their affiliates.
| Name |
Number of Shares of Series E Preferred Stock |
Aggregate Purchase Price |
|||
| Equilon Enterprises LLC dba Shell Oil Products US(1) |
3,584,428 | $ | 30,467,638.00 | ||
| CMEA Ventures Life Sciences 2000, L.P.(2) (3) |
588,236 | 5,000,006.00 | |||
| FirstMark III, L.P. (formerly, Pequot Private Equity Fund III, LP)(4) |
588,235 | 4,999,997.50 | |||
| CTTV Investments LLC |
88,236 | 750,006.00 | |||
| (1) | Chris Streng is one of our directors and is Vice President Finance Manufacturing for Shell Downstream Inc. |
| (2) | Thomas R. Baruch is one of our directors and a managing director of CMEA Ventures. |
| (3) | Includes 36,471 shares held by CMEA Ventures Life Sciences 2000, Civil Law Partnership, an affiliate of CMEA Ventures Life Sciences 2000, L.P. |
| (4) | Includes 72,677 shares held by FirstMark III Offshore Partners, L.P. (formerly, Pequot Offshore Private Equity Partners III, LP), an affiliate of FirstMark III, L.P. |
134
Table of Contents
Issuance of Series D Preferred Stock
In August and October 2006, we issued an aggregate of 10,068,402 shares of our Series D preferred stock at a price per share of approximately $3.97 for an aggregate purchase price of approximately $40.0 million, including cancellation of indebtedness. The table below sets forth the number of shares of Series D preferred stock sold to our directors, executive officers and 5% stockholders and their affiliates.
| Name |
Number of Shares of Series D Preferred Stock |
Aggregate Purchase Price |
|||
| Biomedical Sciences Investment Fund Pte Ltd.(1) |
5,037,783 | $ | 19,999,998.51 | ||
| CMEA Ventures Life Sciences 2000, L.P.(2) (3) |
1,520,180 | 6,035,114.60 | |||
| Equilon Enterprises LLC dba Shell Oil Products US(4) (7) |
1,184,239 | 5,999,998.96 | |||
| FirstMark III, L.P. (formerly, Pequot Private Equity Fund III, |
736,375 | 2,923,408.75 | |||
| Maxygen, Inc.(6) |
254,838 | 1,011,706.86 | |||
| CTTV Investments LLC |
755,668 | 3,000,001.96 | |||
| (1) | Mun Yew Wong is one of our directors and Director (Investments) of EDB Investments Pte Ltd and Bio*One Capital Pte Ltd. |
| (2) | Thomas R. Baruch is one of our directors and a managing director of CMEA Ventures. |
| (3) | Includes 94,223 shares held by CMEA Ventures Life Sciences 2000, Civil Law Partnership, an affiliate of CMEA Ventures Life Sciences 2000, L.P. |
| (4) | Chris Streng is one of our directors and is Vice President Finance Manufacturing for Shell Downstream Inc. |
| (5) | Includes 645,395 shares held by FirstMark III Offshore Partners, L.P. (formerly, Pequot Offshore Private Equity Partners III, LP), an affiliate of FirstMark III, L.P. |
| (6) | James R. Sulat is one of our directors and the Chief Executive Officer and Chief Financial Officer and a director of Maxygen, Inc. |
| (7) | Includes 428,571 shares acquired in November 2007 pursuant to the exercise of a warrant at a price per share of $7.00 per share for an aggregate purchase price of $2,999,997.00. |
2006 Bridge Financing
In May 2006, we sold convertible promissory notes, or the 2006 Notes, to certain of our existing investors in the aggregate principal amount of approximately $4.2 million. The 2006 Notes accrued interest at a rate of 8% per annum and had a maturity date of the earlier of (1) December 31, 2006 or (2) the closing of any consolidation or merger of the Company with or into another corporation or entity or a sale of all or substantially all of our assets. In August 2006, in connection with our Series D preferred stock financing described above, the full principal amount of and accrued but unpaid interest on the 2006 Notes was automatically converted into an aggregate of 1,078,571 shares of our Series D preferred stock at a conversion price equal to the issue price of our Series D preferred stock.
In connection with the 2006 Notes, we issued warrants to purchase an aggregate of 323,569 shares of our Series D preferred stock at an exercise price of $3.97 per share to the purchasers of the 2006 Notes. The warrants may be exercised at any time prior to their respective termination dates, which are the seventh anniversaries of their issue dates.
135
Table of Contents
The table below sets forth the principal amount of the 2006 Notes and the shares of Series D preferred stock issuable upon exercise of the related warrants sold to our directors, executive officers and 5% stockholders and their affiliates.
| Name |
Aggregate Principal Amount of 2006 Notes |
Shares of Series D Preferred Stock Issuable Upon the Exercise of Warrants |
|||
| CMEA Ventures Life Sciences 2000, L.P.(1) (2) (3) |
$ | 1,800,000 | 138,673 | ||
| FirstMark III, L.P. (formerly, Pequot Private Equity Fund III, LP)(4) (5) |
$ | 1,200,000 | 92,448 | ||
| Maxygen, Inc.(6) |
$ | 600,000 | 46,224 | ||
| CTTV Investments LLC |
$ | 600,000 | 46,224 | ||
| (1) | Thomas R. Baruch is one of our directors and a managing director of CMEA Ventures. |
| (2) | Includes $111,565.82 aggregate principal amount invested by CMEA Ventures Life Sciences 2000, Civil Law Partnership, an affiliate of CMEA Ventures Life Sciences 2000, L.P. |
| (3) | Includes 8,595 shares of Series D preferred stock issuable upon the exercise of a warrant held by CMEA Ventures Life Sciences 2000, Civil Law Partnership, an affiliate of CMEA Ventures Life Sciences 2000, L.P. |
| (4) | Includes $148,261.00 aggregate principal amount invested by FirstMark III Offshore Partners, L.P. (formerly, Pequot Offshore Private Equity Partners III, LP), an affiliate of FirstMark III, L.P. |
| (5) | Includes 11,422 shares of Series D preferred stock issuable upon the exercise of a warrant held by FirstMark III Offshore Partners, L.P. (formerly, Pequot Offshore Private Equity Partners III, LP), an affiliate of FirstMark III, L.P. |
| (6) | James R. Sulat is one of our directors and the Chief Executive Officer and Chief Financial Officer and a director of Maxygen, Inc. |
Registration Rights Agreement
We have entered into an investors rights agreement with the purchasers of our outstanding preferred stock and certain holders of common stock and warrants to purchase our common stock and preferred stock, including entities with which certain of our directors are affiliated. Additionally, in connection with our acquisition of Jülich Fine Chemicals GmbH we entered into a registration rights agreement with certain stockholders of Jülich who acquired shares of our common stock in connection with the acquisition. As of September 30, 2009, the holders of 38,418,542 shares of our common stock, including the shares of common stock issuable upon the automatic conversion of our preferred stock and shares of common stock issued upon exercise of warrants, are entitled to rights with respect to the registration of their shares under the Securities Act. For a more detailed description of these registration rights, see Description of Capital Stock Registration Rights.
Other Transactions
In March 2002, we licensed core enabling technology from Maxygen and commenced operations. The license agreement was amended in September 2002, October 2002 and August 2006. See Business License Agreement with Maxygen.
In November 2006, we entered into a research agreement and license agreement with Shell. In November 2007, we entered into a new collaboration under an amended and restated collaborative research agreement and an amended and restated license agreement. Both of these agreements were further amended in March 2009. See Business Strategic Collaborations Shell and Other Biofuels Partners.
136
Table of Contents
We have entered into change of control agreements with certain of our executive officers that, among other things, provide for certain severance and change of control benefits. For a description of these agreements, see Management Change in Control Agreements.
We have granted stock options to our executive officers and certain of our directors. For a description of these options, see Management Grants of Plan-Based Awards in 2008 Table.
In December 2009, we entered into a consulting agreement with Alexander A. Karsner, one of our directors. Under the consulting agreement, Mr. Karsner agreed to provide certain strategic advisory services related to the energy industry and government relations, as requested by us from time to time, in exchange for cash compensation of $120,000 per year, payable on a quarterly basis. Pursuant to the consulting agreement, we also granted Mr. Karsner an option to purchase 100,000 shares of our common stock pursuant to our 2002 Stock Plan, which vests monthly as to 1/48th of the total shares subject to the option, provided that Mr. Karsner continues to provide services to us under the consulting agreement. The consulting agreement has a term of four years, but is terminable at any time by either party.
We have entered into indemnification agreements with each of our directors, and will enter into new indemnification agreements with each of our current directors, officers, and certain employees before the completion of this offering. See Management Limitation on Liability and Indemnification Matters.
Policies and Procedures for Related Party Transactions
Our board of directors intends to adopt a written related person transaction policy to set forth the policies and procedures for the review and approval or ratification of related person transactions. This policy will cover, with certain exceptions set forth in Item 404 of Regulation S-K under the Securities Act, any transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships in which we were or are to be a participant, the amount involved exceeds $120,000, and a related person had or will have a direct or indirect material interest, including, without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness, and employment by us of a related person.
137
Table of Contents
PRINCIPAL AND SELLING STOCKHOLDERS
The following table sets forth information about the beneficial ownership of our common stock at September 30, 2009 (based on the total number of shares of common stock outstanding on September 30, 2009, as adjusted to reflect the conversion of all shares of our outstanding preferred stock and assuming the sale of shares of our common stock in this offering) as adjusted to reflect the sale of the shares of common stock in this offering for:
| | each person known to us to be the beneficial owner of more than 5% of our common stock; |
| | each named executive officer and each director; |
| | all of our executive officers and directors as a group; and |
| | each selling stockholder. |
Unless otherwise noted below, the address of each beneficial owner listed on the table is c/o Codexis, Inc., 200 Penobscot Drive, Redwood City, CA 94063. We have determined beneficial ownership in accordance with the rules of the SEC. Except as indicated by the footnotes below, we believe, based on the information furnished to us, that the persons and entities named in the tables below have sole voting and investment power with respect to all shares of common stock that they beneficially own, subject to applicable community property laws.
In computing the number of shares of common stock beneficially owned by a person after the offering, we have assumed the issuance of 37,624,216 shares of common stock to holders of our preferred stock upon the closing of this offering as a cumulative dividend, pursuant to the terms of our certificate of incorporation.
In computing the number of shares of common stock beneficially owned by a person and the percentage ownership of that person, we deemed outstanding shares of common stock subject to options or warrants held by that person that are currently exercisable or exercisable within 60 days of September 30, 2009. We did not deem these shares outstanding, however, for the purpose of computing the percentage ownership of any other person.
138
Table of Contents
We have based our calculation of the percentage of beneficial ownership prior to the offering on 41,599,768 shares of common stock outstanding on September 30, 2009 (as adjusted to reflect at that date the conversion of all shares of our preferred stock outstanding into 37,624,216 shares of common stock). We have based our calculation of the percentage of beneficial ownership after the offering on shares of our common stock outstanding immediately after the completion of this offering.
| Number of Shares Beneficially Owned |
Shares Being Offered |
Percentage of Shares Beneficially Owned |
|||||||||
| Name and Address of Beneficial Owner |
Prior to the Offering |
After the Offering |
Prior to the Offering |
After the Offering |
|||||||
| 5% Stockholders: |
|||||||||||
| Maxygen, Inc.(1) |
8,981,888 | 21.57 | % | ||||||||
| Equilon Enterprises LLC dba Shell Oil Products US |
8,298,078 | 19.95 | % | ||||||||
| Biomedical Sciences Investment Fund Pte Ltd(2) |
5,037,783 | 12.11 | % | ||||||||
| Entities affiliated with CMEA Ventures(3) |
4,515,397 | 10.82 | % | ||||||||
| Entities affiliated with FirstMark Capital (formerly, Pequot Capital Management)(4) |
4,009,411 | 9.62 | % | ||||||||
| CTTV Investments LLC(5) |
2,510,348 | 6.03 | % | ||||||||
| Executive Officers and Directors: |
|||||||||||
| Alan Shaw(6) |
1,658,177 | 3.85 | % | ||||||||
| Robert J. Lawson |
| | |||||||||
| David L. Anton(7) |
62,499 | * | |||||||||
| Joseph J. Sarret(8) |
179,508 | * | |||||||||
| Douglas T. Sheehy(9) |
144,602 | * | |||||||||
| Thomas R. Baruch(10) |
4,515,397 | 10.82 | % | ||||||||
| Alexander A. Karsner |
| | |||||||||
| Bernard J. Kelley(11) |
145,000 | * | |||||||||
| Bruce Pasternack(12) |
75,000 | * | |||||||||
| Chris Streng |
| | |||||||||
| James R. Sulat(13) |
8,981,888 | 21.57 | % | ||||||||
| Dennis P. Wolf(14) |
75,000 | * | |||||||||
| Mun Yew Wong |
| | |||||||||
| Robert S. Breuil(15) |
442,824 | 1.05 | % | ||||||||
| Peter Seufer-Wasserthal(16) |
186,700 | * | |||||||||
| All executive officers and directors as a group (15 persons) |
16,466,595 | 36.95 | % | ||||||||
| Other Selling Stockholders: |
|||||||||||
| * | Represents beneficial ownership of less than 1% of the outstanding shares of our common stock. |
| (1) | Includes 46,224 shares that may be acquired pursuant to the exercise of a warrant held prior to this offering by Maxygen, Inc. |
| (2) | Biomedical Sciences Investment Fund Pte Ltd, or Bio*One, is wholly-owned by EDB Investments Pte Ltd, which is wholly-owned by the Economic Development Board of Singapore. No individual has beneficial ownership over shares held by Bio*One. Voting and investment decisions relating to these securities are made by the board of directors of Bio*One, which is currently comprised of Ms. Chu Swee Yeok and Mr. Beh Kian Teik. The board of directors of Bio*One acts by majority vote and no board member may act individually to vote or sell these securities. |
| (3) | Includes (i) 4,105,438 shares and 130,078 shares that may be acquired pursuant to the exercise of a warrant held prior to this offering by CMEA Ventures Life Sciences 2000, L.P. and (ii) 271,286 shares and 8,595 shares that may be acquired pursuant to the exercise of a warrant held prior to this |
139
Table of Contents
| offering by CMEA Ventures Life Sciences 2000, Civil Law Partnership. CMEA Ventures LS Management 2000, L.P. is the general partner of CMEA Ventures Life Sciences 2000, L.P. and the managing limited partner of CMEA Ventures Life Sciences 2000, Civil Law Partnership. David Collier, Karl Handelsman and Thomas R. Baruch are the general partners of CMEA Ventures LS Management 2000, L.P. and as such, have voting and dispositive power over these shares. Each disclaims beneficial ownership of the shares and warrants held by these entities except to the extent of any pecuniary interest therein. |
| (4) | Includes (i) 3,433,018 shares and 81,026 shares that may be acquired pursuant to the exercise of a warrant held prior to this offering by FirstMark III, L.P. and (ii) 483,945 shares and 11,422 shares that may be acquired pursuant to the exercise of a warrant held prior to this offering by FirstMark III Offshore Partners, L.P. FirstMark Capital, LLC, or FirstMark, is the investment manager/advisor of, and exercises sole investment discretion over, FirstMark III, L.P. and FirstMark III Offshore Partners, L.P., and as such, has voting and dispositive power over these shares. Lawrence D. Lenihan, Jr. is the chief executive officer and a managing member of FirstMark, and Gerald A. Poch is the chairman and a managing member of FirstMark. As such, each of Mr. Lenihan and Mr. Poch have voting and dispositive power over these shares. Each of Mr. Lenihan and Mr. Poch disclaim beneficial ownership of the shares and shares underlying warrants held by these entities, except to the extent of each of his pecuniary interest therein. |
| (5) | Includes 46,224 shares that may be acquired pursuant to the exercise of a warrant held prior to this offering by CTTV Investments LLC. CTTV Investments LLC, or CTTV, is wholly owned by Chevron Technology Ventures LLC, or Chevron Technology Ventures, and the ultimate beneficial owner of shares held by CTTV is Chevron Corporation. No individual has beneficial ownership of the shares held by CTTV. Voting and investment decisions relating the securities owned by CTTV are made by an investment committee of the venture capital business unit of Chevron Technology Ventures, which consists of the Corporate Vice President and Chief Technology Officer, Corporate Controller and General Manager of Mergers and Acquisitions, with such positions currently being held by John McDonald, Mark Humphrey and Mark Menke, respectively. |
| (6) | Includes (i) 71,302 shares held by Alan Shaw, Trustee of The Alan Shaw 2008 Annuity Trust, dated June 20, 2008, (ii) 66,198 shares held by The Shaw Living Trust Agreement and (iii) 1,520,677 shares issuable pursuant to stock options exercisable within 60 days of September 30, 2009. |
| (7) | Includes 62,499 shares issuable pursuant to stock options exercisable within 60 days of September 30, 2009. |
| (8) | Includes (i) 20,000 shares held by Joseph Sarret as Trustee UTD 5/30/00 and (ii) 159,508 shares issuable pursuant to stock options exercisable within 60 days of September 30, 2009. |
| (9) | Includes 144,602 shares issuable pursuant to stock options exercisable within 60 days of September 30, 2009. |
| (10) | Includes (i) 4,105,438 shares and 130,078 shares that may be acquired pursuant to the exercise of a warrant held prior to this offering by CMEA Ventures Life Sciences 2000, L.P. and (ii) 271,286 shares and 8,595 shares that may be acquired pursuant to the exercise of a warrant held prior to this offering by CMEA Ventures Life Sciences 2000, Civil Law Partnership. CMEA Ventures LS Management 2000, L.P. is the general partner of CMEA Ventures Life Sciences 2000, L.P. and the managing limited partner of CMEA Ventures Life Sciences 2000, Civil Law Partnership. Mr. Baruch is a general partner of CMEA Ventures LS Management 2000, L.P. and as such, has voting and dispositive power over these shares. Mr. Baruch disclaims beneficial ownership of the shares and warrants held by these entities except to the extent of his pecuniary interest therein. |
140
Table of Contents
| (11) | Includes 107,500 shares issuable pursuant to stock options exercisable within 60 days of September 30, 2009. Such options are vested as to 61,666 shares, and the remaining 45,834 shares, if the options are exercised, would be subject to a right of repurchase within 60 days of September 30, 2009, at the original option exercise price, in the event Mr. Kelley ceases to provide services to us. The option exercise prices range from $0.70 to $7.00 per share. |
| (12) | Includes 75,000 shares issuable pursuant to stock options exercisable within 60 days of September 30, 2009. Such options are vested as to 25,520 shares, and the remaining 49,480 shares, if the options are exercised, would be subject to a right of repurchase within 60 days of September 30, 2009, at the original option exercise price, in the event Mr. Pasternack ceases to provide services to us. The option exercise prices range from $4.47 to $7.00 per share. |
| (13) | Includes 8,935,664 shares and 46,224 shares that may be acquired pursuant to the exercise of a warrant held prior to this offering by Maxygen, Inc. Mr. Sulat is the Chief Executive Officer, Chief Financial Officer and a member of the board of directors of Maxygen and may be deemed to be the beneficial owner of our securities held by Maxygen. Mr. Sulat disclaims beneficial ownership of all our securities held by Maxygen, except to the extent of his pecuniary interest therein. Mr. Sulat will resign from our board of directors in connection with the closing of our initial public offering. |
| (14) | Includes 75,000 shares issuable pursuant to stock options exercisable within 60 days of September 30, 2009. Such options are vested as to 23,437 shares, and the remaining 51,563 shares, if the options are exercised, would be subject to a right of repurchase within 60 days of September 30, 2009, at the original option exercise price, in the event Mr. Wolf ceases to provide services to us. The option exercise prices range from $4.97 to $7.00 per share. |
| (15) | Includes 442,824 shares issuable pursuant to stock options exercisable within 60 days of September 30, 2009. |
| (16) | Includes 186,700 shares issuable pursuant to stock options exercisable within 60 days of September 30, 2009. |
141
Table of Contents
General
Upon the completion of this offering, we will have authorized under our amended and restated certificate of incorporation shares of common stock, $0.0001 par value per share, and shares of preferred stock, $ par value per share. The following information assumes the filing of our amended and restated certificate of incorporation and the conversion of all outstanding shares of our preferred stock into shares of common stock upon the completion of this offering.
As of September 30, 2009, there were outstanding:
| | 41,599,768 shares of our common stock held by approximately 109 stockholders; and |
| | 10,962,854 shares of our common stock issuable upon exercise of outstanding stock options. |
The following description of our capital stock and provisions of our amended and restated certificate of incorporation and amended and restated bylaws to be in effect upon the completion of this offering are summaries. Copies of these documents have been filed with the SEC as exhibits to our registration statement, of which this prospectus forms a part. The descriptions of the common stock and preferred stock reflect changes to our capital structure that will occur upon the closing of this offering. Currently, there is no established public trading market for our common stock.
Common Stock
Voting Rights
Each holder of our common stock is entitled to one vote for each share on all matters submitted to a vote of the stockholders, including the election of directors. Our stockholders do not have cumulative voting rights in the election of directors. Accordingly, holders of a majority of the voting shares are able to elect all of the directors.
Dividends
Subject to preferences that may be applicable to any then outstanding preferred stock, holders of our common stock are entitled to receive dividends, if any, as may be declared from time to time by our board of directors out of legally available funds.
Liquidation
In the event of our liquidation, dissolution or winding up, holders of our common stock will be entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of all of our debts and other liabilities and the satisfaction of any liquidation preference granted to the holders of any then outstanding shares of preferred stock.
Rights and Preferences
Holders of our common stock have no preemptive, conversion, subscription or other rights, and there are no redemption or sinking fund provisions applicable to our common stock. The rights, preferences and privileges of the holders of our common stock are subject to and may be adversely affected by the rights of the holders of shares of any series of our preferred stock that we may designate in the future.
Preferred Stock
Upon the completion of this offering, our board of directors will have the authority, without further action by our stockholders, to issue up to shares of preferred stock in one or more series and to fix the rights, preferences, privileges and restrictions thereof. These rights, preferences and privileges could
142
Table of Contents
include dividend rights, conversion rights, voting rights, terms of redemption, liquidation preferences, sinking fund terms and the number of shares constituting any series or the designation of such series, any or all of which may be greater than the rights of common stock. The issuance of our preferred stock could adversely affect the voting power of holders of common stock and the likelihood that such holders will receive dividend payments and payments upon liquidation. In addition, the issuance of preferred stock could have the effect of delaying, deferring or preventing a change of control of our company or other corporate action. Upon completion of this offering, no shares of preferred stock will be outstanding, and we have no present plan to issue any shares of preferred stock.
Warrants
The following table sets forth information about outstanding warrants to purchase shares of our stock as of September 30, 2009. Upon completion of this offering, the warrants to purchase shares of our Series D preferred stock will automatically convert into warrants to purchase our common stock.
| Class of Stock |
Number of Shares | Exercise Price/Share | Expiration Date | ||||
| Common |
46,176 | $ | 0.40 | 02/12/2011 | |||
| Common |
9,100 | 0.70 | 10/25/2012 | ||||
| Common |
3,577 | 8.30 | 02/09/2016 | ||||
| Series D preferred stock |
323,569 | 3.97 | 05/25/2013 | ||||
| Series D preferred stock |
109,091 | 5.50 | 09/28/2017 | ||||
Registration Rights
We are party to an investors agreement which provides that holders of our preferred stock and our founding stockholder, Maxygen, have the right to demand that we file a registration statement or request that their shares be covered by a registration statement that we are otherwise filing. In the event that we propose to register any of our securities under the Securities Act, either for our own account or for the account of other security holders, these holders are entitled to notice of such registration and are entitled to certain piggyback registration rights allowing the holder to include their common stock in such registration, subject to certain marketing and other limitations. Pursuant to the investors rights agreement, the holders of common stock issuable upon conversion of our preferred stock have the right upon the earlier of 180 days after the completion of this offering and March 4, 2012 to require us, on not more than two occasions, to file a registration statement under the Securities Act in order to register the resale of their shares of common stock with an anticipated aggregate offering price, net of underwriting discounts and commissions, of at least ten million dollars. We may, in certain circumstances, defer such registrations and any underwriters will have the right, subject to certain limitations, to limit the number of shares included in such registrations. Further, these holders may require us to register the resale of all or a portion of their shares on a registration statement on Form S-3 once we are eligible to use Form S-3, subject to certain conditions and limitations. In an underwritten offering, the underwriter, has the right, subject to specified conditions, to limit the number of registrable securities such holders may include. Additionally, the holders of registration rights have waived their rights to include any of their shares in this offering prior to the completion of this offering.
In connection with our acquisition of Jülich Fine Chemicals GmbH in February 2005, we entered into a registration rights agreement with certain stockholders of Jülich who acquired shares of our common stock in connection with the acquisition. If we propose to register any of our securities under the Securities Act, these stockholders are entitled to notice of such registration and are entitled to certain piggyback registration rights allowing the holder to include their common stock in such registration, subject to certain marketing and other limitations. In an underwritten offering, the underwriter, has the right, subject to specified conditions, to limit the number of registrable securities such holders may include. The holders of these registration rights have waived their rights to include any of their shares in this offering prior to the completion of this offering.
143
Table of Contents
Anti-Takeover Provisions
Certificate of Incorporation and Bylaws to be in Effect Upon the Completion of this Offering
Our amended and restated certificate of incorporation to be in effect upon the completion of this offering will provide for our board of directors to be divided into three classes, with staggered three-year terms. Only one class of directors will be elected at each annual meeting of our stockholders, with the other classes continuing for the remainder of their respective three-year terms. Because our stockholders do not have cumulative voting rights, our stockholders holding a majority of the shares of common stock outstanding will be able to elect all of our directors. Our amended and restated certificate of incorporation and amended and restated bylaws to be effective upon the completion of this offering will provide that all stockholder action must be effected at a duly called meeting of stockholders and not by a consent in writing, and that only our board of directors, chairman of the board, chief executive officer, or president (in the absence of a chief executive officer) may call a special meeting of stockholders.
Our amended and restated certificate of incorporation will require a 66 2/3% stockholder vote for the amendment, repeal or modification of certain provisions of our amended and restated certificate of incorporation and amended and restated bylaws relating to the classification of our board of directors, the requirement that stockholder actions be effected at a duly called meeting, and the designated parties entitled to call a special meeting of the stockholders. The combination of the classification of our board of directors, the lack of cumulative voting and the 66 2/3% stockholder voting requirements will make it more difficult for our existing stockholders to replace our board of directors as well as for another party to obtain control of us by replacing our board of directors. Because our board of directors has the power to retain and discharge our officers, these provisions could also make it more difficult for existing stockholders or another party to effect a change in management. In addition, the authorization of undesignated preferred stock makes it possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to change our control.
These provisions may have the effect of deterring hostile takeovers or delaying changes in our control or management. These provisions are intended to enhance the likelihood of continued stability in the composition of our board of directors and its policies and to discourage certain types of transactions that may involve an actual or threatened acquisition of us. These provisions are designed to reduce our vulnerability to an unsolicited acquisition proposal. The provisions also are intended to discourage certain tactics that may be used in proxy fights. However, such provisions could have the effect of discouraging others from making tender offers for our shares and, as a consequence, they also may inhibit fluctuations in the market price of our shares that could result from actual or rumored takeover attempts. Such provisions may also have the effect of preventing changes in our management.
Section 203 of the Delaware General Corporation Law
We are subject to Section 203 of the Delaware General Corporation Law, which prohibits a Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years after the date that such stockholder became an interested stockholder, with the following exceptions:
| | before such date, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested holder; |
| | upon completion of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction began, excluding for purposes of determining the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder) those shares owned (i) by persons who are directors and also officers and (ii) employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
144
Table of Contents
| | on or after such date, the business combination is approved by the board of directors and authorized at an annual or special meeting of the stockholders, and not by written consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock that is not owned by the interested stockholder. |
In general, Section 203 defines business combination to include the following:
| | any merger or consolidation involving the corporation and the interested stockholder; |
| | any sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested stockholder; |
| | subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; |
| | any transaction involving the corporation that has the effect of increasing the proportionate share of the stock or any class or series of the corporation beneficially owned by the interested stockholder; or |
| | the receipt by the interested stockholder of the benefit of any loss, advances, guarantees, pledges or other financial benefits by or through the corporation. |
In general, Section 203 defines an interested stockholder as an entity or person who, together with the persons affiliates and associates, beneficially owns, or is an affiliate or associate of the corporation and within three years prior to the time of determination of interested stockholder status did own, 15% or more of the outstanding voting stock of the corporation.
Limitations of Liability and Indemnification Matters
For an in depth discussion of liability and indemnification, please see Management Limitation on Liability and Indemnification Matters.
The Nasdaq Global Market Listing
We have applied to have our common stock approved for listing on The Nasdaq Global Market under the symbol CDXS.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is American Stock Transfer & Trust Company, LLC.
145
Table of Contents
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no public market for our common stock. Future sales of our common stock in the public market, or the availability of such shares for sale in the public market, could adversely affect market prices prevailing from time to time. As described below, only a limited number of shares will be available for sale shortly after this offering due to contractual and legal restrictions on resale. Nevertheless, sales of our common stock in the public market after such restrictions lapse, or the perception that those sales may occur, could adversely affect the prevailing market price at such time and our ability to raise equity capital in the future.
Based on the number of shares of common stock outstanding as of September 30, 2009, upon completion of this offering, shares of common stock will be outstanding, assuming no exercise of the underwriters option to purchase additional shares and no exercise of options or warrants. All of the shares sold by us and the selling stockholders in this offering will be freely tradable unless purchased by our affiliates. The remaining 41,599,768 shares of common stock outstanding after this offering will be restricted as a result of securities laws or lock-up agreements as described below. Following the expiration of the lock-up period, all shares will be eligible for resale in compliance with Rule 144 or Rule 701 to the extent such shares have been released from any repurchase option that we may hold. Restricted securities as defined under Rule 144 were issued and sold by us in reliance on exemptions from the registration requirements of the Securities Act. These shares may be sold in the public market only if registered or pursuant to an exemption from registration, such as Rule 144 or Rule 701 under the Securities Act.
Rule 144
In general, under Rule 144 of the Securities Act, as in effect on the date of this prospectus, a person (or persons whose shares are aggregated) who has beneficially owned restricted stock for at least six months, will be entitled to sell in any three-month period a number of shares that does not exceed the greater of:
| | 1% of the number of shares of common stock then outstanding ( shares immediately after this offering or shares if the underwriters option to purchase additional shares is exercised in full); or |
| | the average weekly trading volume of our common stock on The Nasdaq Global Market during the four calendar weeks immediately preceding the date on which the notice of sale is filed with the SEC. |
Sales pursuant to Rule 144 are subject to requirements relating to manner of sale, notice and availability of current public information about us. A person (or persons whose shares are aggregated) who is not deemed to be an affiliate of ours for 90 days preceding a sale, and who has beneficially owned restricted stock for at least one year is entitled to sell such shares without complying with the manner of sale, public information, volume limitation or notice provisions of Rule 144. Rule 144 will not be available to any stockholders until we have been subject to the reporting requirements of the Exchange Act for 90 days.
Rule 701
Rule 701 under the Securities Act, as in effect on the date of this prospectus, permits resales of shares in reliance upon Rule 144 but without compliance with certain restrictions of Rule 144, including the holding period requirement. Most of our employees, executive officers or directors who purchased shares under a written compensatory plan or contract may be entitled to rely on the resale provisions of Rule 701, but all holders of Rule 701 shares are required to wait until 90 days after the date of this prospectus before selling their shares. However, substantially all Rule 701 shares are subject to lock-up agreements as described below and under Underwriting included elsewhere in this prospectus and will become eligible for sale upon the expiration of the restrictions set forth in those agreements.
146
Table of Contents
Lock-up Agreements
We, along with our directors, executive officers and substantially all of our other security holders, including selling stockholders, have agreed with the underwriters that for a period of 180 days following the date of this prospectus, we or they will not offer, sell, contract to sell, pledge, or otherwise dispose of, directly or indirectly, any shares of common stock or any securities convertible into or exercisable or exchangeable for shares of common stock, or enter into any swap, hedge or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of the common stock whether any of these transactions are to be settled by delivery of our common stock or other securities, in cash or otherwise, or publicly disclose the intention to make any offer, sale, pledge or disposition, or to enter into any transaction, swap, hedge or other arrangement, subject to specified exceptions. Credit Suisse Securities (USA) LLC and Goldman, Sachs & Co. may, in their sole discretion, at any time without prior notice, release all or any portion of the shares from the restrictions in any such agreement.
The 180-day restricted period described in the preceding paragraph will be extended if:
| | during the last 17 days of the 180-day restricted period we issue an earnings release or material news or a material event relating to us occurs; or |
| | prior to the expiration of the 180-day restricted period, we announce that we will release earnings results during the 16-day period beginning on the last day of the 180-day period, |
in which case the restrictions described in the preceding paragraph will continue to apply until the expiration of the 18-day period beginning on the issuance of the release or the occurrence of the material news or material event, unless such extension is waived, in writing, by Credit Suisse Securities (USA) LLC and Goldman, Sachs & Co. on behalf of the underwriters.
Registration Rights
We are party to an investor rights agreement which provides that holders of our preferred stock and our founding stockholders have the right to demand that we file a registration statement or request that their shares be covered by a registration statement that we are otherwise filing. We are also party to a registration rights agreement with certain former stockholders of Jülich Fine Chemicals GmbH, which we acquired in February 2005, who are entitled to certain piggyback registration rights. See Description of Capital Stock Registration Rights. Except for shares purchased by affiliates, registration of their shares under the Securities Act would result in these shares becoming freely tradable without restriction under the Securities Act immediately upon effectiveness of the registration, subject to the expiration of the lock-up period and to the extent such shares have been released from any repurchase option that we may hold.
Stock Plans
As soon as practicable after the completion of this offering, we intend to file a Form S-8 registration statement under the Securities Act to register shares of our common stock subject to options outstanding or reserved for issuance under our 2002 Stock Plan and our 2008 Incentive Award Plan. This registration statement will become effective immediately upon filing, and shares covered by this registration statement will thereupon be eligible for sale in the public markets, subject to Rule 144 limitations applicable to affiliates and any lock-up agreements. For a more complete discussion of our stock plans, see Management Employee Benefit and Stock Plans.
147
Table of Contents
MATERIAL UNITED STATES FEDERAL INCOME TAX
CONSEQUENCES TO NON-U.S. HOLDERS
The following is a summary of material United States federal income tax consequences to non-U.S. holders (as defined below) of the acquisition, ownership and disposition of our common stock issued pursuant to this offering. This discussion is not a complete analysis of all of the potential United States federal income tax consequences relating thereto, nor does it address any estate and gift tax consequences or any tax consequences arising under any state, local or foreign tax laws, or any other United States federal tax laws. This discussion is based on the Internal Revenue Code of 1986, as amended, or the Code, Treasury Regulations promulgated thereunder, judicial decisions, and published rulings and administrative pronouncements of the Internal Revenue Service, or IRS, all as in effect as of the date of this offering. These authorities may change, possibly retroactively, resulting in United States federal income tax consequences different from those discussed below. No ruling has been or will be sought from the IRS with respect to the matters discussed below, and there can be no assurance that the IRS will not take a contrary position regarding the tax consequences of the acquisition, ownership or disposition of our common stock, or that any such contrary position would not be sustained by a court.
This discussion is limited to non-U.S. holders who purchase our common stock issued pursuant to this offering and who hold our common stock as a capital asset within the meaning of Section 1221 of the Code (for example, property held for investment). This discussion does not address all of the United States federal income tax consequences that may be relevant to a particular holder in light of such holders particular circumstances. This discussion also does not consider any specific facts or circumstances that may be relevant to holders subject to special rules under the United States federal income tax laws, including, without limitation:
| | U.S. expatriates or former long-term residents of the United States; |
| | partnerships or other pass-through entities; |
| | real estate investment trusts; |
| | regulated investment companies; |
| | controlled foreign corporations, passive foreign investment companies corporations that accumulate earnings to avoid United States federal income tax; |
| | banks, insurance companies, or other financial institutions; |
| | brokers, dealers, or traders in securities, commodities or currencies; |
| | tax-exempt organizations; |
| | tax-qualified retirement plans; |
| | persons subject to the alternative minimum tax; or |
| | persons holding our common stock as part of a hedging or conversion transaction or straddle, or a constructive sale, or other risk reduction strategy. |
PROSPECTIVE INVESTORS ARE URGED TO CONSULT THEIR TAX ADVISORS REGARDING THE PARTICULAR UNITED STATES FEDERAL INCOME TAX CONSEQUENCES TO THEM OF ACQUIRING, OWNING AND DISPOSING OF OUR COMMON STOCK, AS WELL AS ANY TAX CONSEQUENCES ARISING UNDER ANY STATE, LOCAL OR FOREIGN TAX LAWS AND ANY OTHER UNITED STATES FEDERAL TAX LAWS.
148
Table of Contents
Definition of Non-U.S. Holder
For purposes of this discussion, a non-U.S. holder is any beneficial owner of our common stock that is not a U.S. person or a partnership (or other entity treated as a partnership) for United States federal income tax purposes. A U.S. person is any of the following:
| | an individual citizen or resident of the United States; |
| | a corporation (or other entity treated as a corporation for United States federal income tax purposes) created or organized under the laws of the United States, any state therein or the District of Columbia; |
| | an estate the income of which is subject to United States federal income tax regardless of its source; or |
| | a trust (1) the administration of which is subject to the primary supervision of a United States court and all substantial decisions of which are controlled by one or more United States persons or (2) that has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person. |
Distributions on Our Common Stock
If we make cash or other property distributions on our common stock, such distributions will constitute dividends for United States federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under United States federal income tax principles. Amounts not treated as dividends for United States federal income tax purposes will constitute a return of capital and will first be applied against and reduce a holders adjusted tax basis in the common stock, but not below zero. Distributions in excess of our current and accumulated earnings and profits and in excess of a non-U.S. holders tax basis in its shares will be treated as gain realized on the sale or other disposition of the common stock and will be treated as described under Gain on Disposition of Our Common Stock below.
Dividends paid to a non-U.S. holder of our common stock will be subject to United States federal withholding tax at a rate of 30% of the gross amount of the dividends, or such lower rate specified by an applicable income tax treaty. To receive the benefit of a reduced treaty rate, a non-U.S. holder must furnish to us or our paying agent a valid IRS Form W-8BEN (or applicable successor form) certifying such holders qualification for the reduced rate. This certification must be provided to us or our paying agent prior to the payment of dividends and must be updated periodically. Non-U.S. holders that do not timely provide us or our paying agent with the required certification, but which qualify for a reduced treaty rate, may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS.
If a non-U.S. holder holds our common stock in connection with the conduct of a trade or business in the United States, and dividends paid on the common stock are effectively connected with such holders United States trade or business, and, if required by an applicable income tax treaty, attributable to a permanent establishment maintained by the non-U.S. holder in the United States, the non-U.S. holder will be exempt from United States federal withholding tax. To claim the exemption, the non-U.S. holder must furnish to us or our paying agent a properly executed IRS Form W-8ECI (or applicable successor form).
Any dividends paid on our common stock that are effectively connected with a non-U.S. holders United States trade or business (and if required by an applicable income tax treaty, attributable to a permanent establishment maintained by the non-U.S. holder in the United States) will be subject to United States federal income tax on a net income basis at the regular graduated United States federal income tax rates in much the same manner as if such holder were a resident of the United States, unless an
149
Table of Contents
applicable income tax treaty provides otherwise. A non-U.S. holder that is a foreign corporation also may be subject to a branch profits tax equal to 30% (or such lower rate specified by an applicable income tax treaty) of a portion of its effectively connected earnings and profits for the taxable year, as adjusted for certain items. Non-U.S. holders are urged to consult any applicable income tax treaties that may provide for different rules.
A non-U.S. holder who claims the benefit of an applicable income tax treaty will be required to satisfy applicable certification and other requirements prior to the distribution date. Non-U.S. holders should consult their tax advisors regarding their entitlement to benefits under a relevant income tax treaty.
Gain on Disposition of Our Common Stock
A non-U.S. holder will not be subject to United States federal income tax on any gain realized upon the sale or other disposition of our common stock, unless:
| | the gain is effectively connected with the non-U.S. holders conduct of a trade or business in the United States, and if required by an applicable income tax treaty, attributable to a permanent establishment maintained by the non-U.S. holder in the United States; |
| | the non-U.S. holder is a nonresident alien individual present in the United States for 183 days or more during the calendar year of the disposition, and certain other requirements are met; or |
| | our common stock constitutes a United States real property interest by reason of our status as a United States real property holding corporation, or USRPHC, for United States federal income tax purposes at any time within the shorter of the five-year period preceding the disposition or the non-U.S. holders holding period for our common stock. The determination of whether we are a USRPHC depends on the fair market value of our United States real property interests relative to the fair market value of our other trade or business assets and our foreign real property interests. |
We believe we are not currently and do not anticipate becoming a USRPHC for United States federal income tax purposes. Even if we become a USRPHC, however, so long as our common stock is regularly traded on an established securities market, such common stock will be treated as U.S. real property interests only if the non-U.S. holder actually or constructively holds more than 5% of our common stock.
Unless an applicable income tax treaty provides otherwise, gain described in the first bullet point above will be subject to United States federal income tax on a net income basis at the regular graduated United States federal income tax rates in much the same manner as if such holder were a resident of the United States. Further, non-U.S. holders that are foreign corporations also may be subject to a branch profits tax equal to 30% (or such lower rate specified by an applicable income tax treaty) of a portion of its effectively connected earnings and profits for the taxable year, as adjusted for certain items.
Gain described in the second bullet point above will be subject to United States federal income tax at a flat 30% rate (or such lower rate specified by an applicable income tax treaty), but may be offset by United States source capital losses (even though the individual is not considered a resident of the United States).
Non-U.S. holders are urged to consult any applicable income tax treaties that may provide for different rules.
Information Reporting and Backup Withholding
We must report annually to the IRS and to each non-U.S. holder the amount of distributions on our common stock paid to such holder and the amount of any tax withheld with respect to those distributions. These information reporting requirements apply even if no withholding was required because the distributions were effectively connected with the holders conduct of a United States trade or business, or withholding was reduced or eliminated by an applicable income tax treaty. This information also may be made available under a specific treaty or agreement with the tax authorities in the country in which the
150
Table of Contents
non-U.S. holder resides or is established. Backup withholding, currently at a 28% rate, may apply to distribution payments to a non-U.S. holder of our common stock and information reporting and backup withholding may apply to the payments of the proceeds of a sale of our common stock within the United States or through certain U.S.-related financial intermediaries, unless the non-U.S. holder furnishes to us or our paying agent the required certification as to its non-U.S. status, such as by providing a valid IRS Form W-8BEN or IRS Form W-8ECI, or certain other requirements are met. Notwithstanding the foregoing, backup withholding may apply if either we have or our paying agent has actual knowledge, or reason to know, that the holder is a U.S. person that is not an exempt recipient.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or a credit against a non-U.S. holders United States federal income tax liability, provided the required information is timely furnished to the IRS.
Proposed Legislation
Legislative proposals have been introduced that, if enacted in their current form, would substantially revise some of the rules discussed above, including with respect to certification requirements and information reporting. In the event of non-compliance with the revised certification requirements, withholding tax could be imposed on payments to certain non-U.S. Holders. It cannot be predicted whether, or in what form, these proposals will be enacted. Prospective investors should consult their own tax advisers regarding these proposals.
151
Table of Contents
Under the terms and subject to the conditions contained in an underwriting agreement dated , 2010 we and the selling stockholders have agreed to sell to the underwriters named below, for whom Credit Suisse Securities (USA) LLC, Goldman, Sachs & Co., Piper Jaffray & Co., RBC Capital Markets Corporation and Pacific Crest Securities LLC are acting as representatives, the following respective numbers of shares of common stock:
| Underwriter |
Number of Shares | |
| Credit Suisse Securities (USA) LLC |
||
| Goldman, Sachs & Co. |
||
| Piper Jaffray & Co. |
||
| RBC Capital Markets Corporation |
||
| Pacific Crest Securities LLC |
||
| Total |
||
The underwriting agreement provides that the underwriters are obligated to purchase all the shares of common stock in the offering if any are purchased, other than those shares covered by the option described below. The underwriting agreement also provides that if an underwriter defaults, the purchase commitments of non-defaulting underwriters may be increased or the offering may be terminated.
We have granted to the underwriters a 30-day option to purchase on a pro rata basis up to additional shares of common stock, at the initial public offering price, less the underwriting discounts and commissions.
The underwriters propose to offer the shares of common stock initially at the public offering price on the cover page of this prospectus and to selling group members at that price less a selling concession of $ per share. The underwriters and selling group members may allow a discount of $ per share on sales to other broker/dealers. After the initial public offering the representatives may change the public offering price and concession and discount to broker/dealers. The offering of the shares of common stock by the underwriters is subject to receipt and acceptance to the underwriters right to reject any order in whole or in part.
The following table summarizes the compensation and estimated expenses we and the selling stockholders will pay:
| Per Share | Total | |||||||||||
| No Exercise | Full Exercise | No Exercise | Full Exercise | |||||||||
| Underwriting discounts and commissions paid by us |
$ | $ | $ | $ | ||||||||
| Underwriting discounts and commissions paid by the selling stockholders |
$ | $ | $ | $ | ||||||||
| Expenses payable by us |
$ | $ | $ | $ | ||||||||
| Expenses payable by the selling stockholders |
$ | $ | $ | $ | ||||||||
The representatives have informed us that they do not expect sales to accounts over which the underwriters have discretionary authority to exceed 5% of the shares of common stock being offered.
We have agreed that we will not offer, sell, contract to sell, pledge or otherwise dispose of, directly or indirectly, or file with the SEC a registration statement under the Securities Act relating to, any shares of
152
Table of Contents
our common stock or securities convertible into or exchangeable or exercisable for any shares of our common stock, or publicly disclose the intention to make any offer, sale, pledge, disposition or filing, without the prior written consent of Credit Suisse Securities (USA) LLC and Goldman, Sachs & Co., or the Lead Representatives, for a period of 180 days after the date of this prospectus, except issuances pursuant to the exercise of warrants or employee stock options outstanding on the date hereof or grants of employee stock options pursuant to the terms of a plan in effect on the date hereof. However, in the event that either (1) during the last 17 days of the lock-up period, we release earnings results or material news or a material event relating to us occurs or (2) prior to the expiration of the lock-up period, we announce that we will release earnings results during the 16-day period beginning on the last day of the lock-up period, then in either case the expiration of the lock-up will be extended until the expiration of the 18-day period beginning on the date of the release of the earnings results or the occurrence of the material news or event, as applicable, unless the Lead Representatives waive, in writing, such an extension.
Our officers and directors and holders of substantially all of our outstanding securities have agreed that they will not offer, sell, contract to sell, pledge or otherwise dispose of, directly or indirectly, any shares of our common stock or securities convertible into or exchangeable or exercisable for any shares of our common stock, enter into a transaction that would have the same effect, or enter into any swap, hedge or other arrangement that transfers, in whole or in part, any of the economic consequences of ownership of our common stock, whether any of these transactions are to be settled by delivery of our common stock or other securities, in cash or otherwise, or publicly disclose the intention to make any offer, sale, pledge or disposition, or to enter into any transaction, swap, hedge or other arrangement, without, in each case, the prior written consent of the Lead Representatives for a period of 180 days after the date of this prospectus, except transfers of shares of our common stock or securities convertible into or exchangeable or exercisable for shares of our common stock by will or intestate succession, in connection with a bona fide gift or in distributions or transfers to limited partners, members, affiliates or stockholders of a security holder, provided that in each case the transferee agrees to be subject to the terms of the lock-up. However, in the event that either (1) during the last 17 days of the lock-up period, we release earnings results or material news or a material event relating to us occurs or (2) prior to the expiration of the lock-up period, we announce that we will release earnings results during the 16-day period beginning on the last day of the lock-up period, then in either case the expiration of the lock-up will be extended until the expiration of the 18-day period beginning on the date of the release of the earnings results or the occurrence of the material news or event, as applicable, unless the Lead Representatives waive, in writing, such an extension. Notwithstanding the foregoing, our officers and directors may enter into a written trading plan established pursuant to Rule 10b5-1 of the Exchange Act during the lock-up period, and we may announce the establishment of such a plan, provided that no direct or indirect offers, pledges, sales, contracts to sell, sales of any option or contract to purchase, purchases of any option or contract to sell, grants of any option, right or warrant to purchase, loans, or other transfers or disposals of any shares of our common stock or any securities convertible into or exercisable or exchangeable for shares of our common stock may be effected pursuant to such plan during the lock-up period.
We and the selling stockholders have agreed to indemnify the underwriters against liabilities under the Securities Act, or contribute to payments that the underwriters may be required to make in that respect.
Prior to this offering, there has been no public market for our common stock. The initial public offering price has been negotiated among us, and the selling stockholders and the representatives. The factors to be considered in determining the initial public offering price of the shares of our common stock, in addition to prevailing market conditions, will be our historical performance, estimates of our business potential and earnings prospects, an assessment of our management and the consideration of the above factors in relation to market valuation of companies in related businesses. We have applied to list the shares of our common stock on The Nasdaq Global Market, under the symbol CDXS.
Certain of the underwriters and their respective affiliates have, from time to time, performed, and may in the future perform, various financial advisory and investment banking services for the company, for which they received or will receive customary fees and expenses.
153
Table of Contents
In connection with the offering, the underwriters may purchase and sell shares of our common stock in the open market. These transactions may include short sales, stabilizing transactions and purchases to cover positions created by short sales. Short sales involve the sale by the underwriters of a greater number of shares than they are required to purchase in the offering. Covered short sales are sales made in an amount not greater than the underwriters option to purchase additional shares from us in the offering. The underwriters may close out any covered short position by either exercising their option to purchase additional shares or purchasing shares in the open market. In determining the source of shares to close out the covered short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase additional shares pursuant to the option granted to them. Naked short sales are any sales in excess of such option. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the common stock in the open market after pricing that could adversely affect investors who purchase in the offering. Stabilizing transactions consist of various bids for or purchases of common stock made by the underwriters in the open market prior to the completion of the offering.
The underwriters may also impose a penalty bid. This occurs when a particular underwriter repays to the underwriters a portion of the underwriting discount received by it because the representatives have repurchased shares sold by or for the account of such underwriter in stabilizing or short covering transactions.
Purchases to cover a short position and stabilizing transactions, as well as other purchases by the underwriters for their own accounts, may have the effect of preventing or retarding a decline in the market price of the companys stock, and together with the imposition of the penalty bid, may stabilize, maintain or otherwise affect the market price of the common stock. As a result, the price of the common stock may be higher than the price that otherwise might exist in the open market. If these activities are commenced, they may be discontinued at any time. These transactions may be effected on The Nasdaq Global Market, in the over-the-counter market or otherwise.
A prospectus in electronic format may be made available on the web sites maintained by one or more of the underwriters, or selling group members, if any, participating in this offering and one or more of the underwriters participating in this offering may distribute prospectuses electronically. The representatives may agree to allocate a number of shares to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the underwriters and selling group members that will make internet distributions on the same basis as other allocations.
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a Relevant Member State), each underwriter has represented and agreed that with effect from and including the date on which the Prospectus Directive is implemented in that Relevant Member State (the Relevant Implementation Date) it has not made and will not make an offer of shares to the public in that Relevant Member State prior to the publication of a prospectus in relation to the shares which has been approved by the competent authority in that Relevant Member State or, where appropriate, approved in another Relevant Member State and notified to the competent authority in that Relevant Member State, all in accordance with the Prospectus Directive, except that it may, with effect from and including the Relevant Implementation Date, make an offer of shares to the public in that Relevant Member State at any time:
(a) to legal entities which are authorised or regulated to operate in the financial markets or, if not so authorised or regulated, whose corporate purpose is solely to invest in securities;
(b) to any legal entity which has two or more of (1) an average of at least 250 employees during the last financial year; (2) a total balance sheet of more than 43,000,000 Euros and (3) an annual net turnover of more than 50,000,000 Euros, as shown in its last annual or consolidated accounts;
154
Table of Contents
(c) to fewer than 100 natural or legal persons (other than qualified investors as defined in the Prospectus Directive) subject to obtaining the prior consent of the representatives for any such offer; or
(d) in any other circumstances which do not require the publication by the company of a prospectus pursuant to Article 3 of the Prospectus Directive.
For the purposes of this provision, the expression an offer of shares to the public in relation to any shares in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the shares to be offered so as to enable an investor to decide to purchase or subscribe the shares, as the same may be varied in that Relevant Member State by any measure implementing the Prospectus Directive in that Relevant Member State and the expression Prospectus Directive means Directive 2003/71/EC and includes any relevant implementing measure in each Relevant Member State.
Each underwriter has represented and agreed that:
| | it has only communicated or caused to be communicated and will only communicate or cause to be communicated an invitation or inducement to engage in investment activity (within the meaning of Section 21 of the Financial Services and Market Act 2000 (as amended), or the FSMA) received by it in connection with the issue or sale of the shares in circumstances in which Section 21(1) of the FSMA would not apply to the company; and |
| | it has complied and will comply with all applicable provisions of the FSMA with respect to anything done by it in relation to the shares in, from or otherwise involving the United Kingdom. |
The shares may not be offered or sold by means of any document other than (i) in circumstances which do not constitute an offer to the public within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), or (ii) to professional investors within the meaning of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) and any rules made thereunder, or (iii) in other circumstances which do not result in the document being a prospectus within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), and no advertisement, invitation or document relating to the shares may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the laws of Hong Kong) other than with respect to shares which are or are intended to be disposed of only to persons outside Hong Kong or only to professional investors within the meaning of the Securities and Futures Ordinance (Cap. 571, Laws of Hong Kong) and any rules made thereunder.
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the shares may not be circulated or distributed, nor may the shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore, or the SFA, (ii) to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
Where the shares are subscribed or purchased under Section 275 by a relevant person which is: (a) a corporation (which is not an accredited investor) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or (b) a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary is an accredited investor, shares, debentures and units of shares and debentures of that corporation or the beneficiaries rights and interest in that trust shall not be transferable for six months after
155
Table of Contents
that corporation or that trust has acquired the shares under Section 275 except: (1) to an institutional investor under Section 274 of the SFA or to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA; (2) where no consideration is given for the transfer; or (3) by operation of law.
The securities have not been and will not be registered under the Securities and Exchange Law of Japan (the Securities and Exchange Law) and each underwriter has agreed that it will not offer or sell any securities, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan (which term as used herein means any person resident in Japan, including any corporation or other entity organized under the laws of Japan), or to others for re-offering or resale, directly or indirectly, in Japan or to a resident of Japan, except pursuant to an exemption from the registration requirements of, and otherwise in compliance with, the Securities and Exchange Law and any other applicable laws, regulations and ministerial guidelines of Japan.
156
Table of Contents
Resale Restrictions
The distribution of our common stock in Canada is being made only on a private placement basis exempt from the requirement that we prepare and file a prospectus with the securities regulatory authorities in each province where trades of common stock are made. Any resale of our common stock in Canada must be made under applicable securities laws which will vary depending on the relevant jurisdiction, and which may require resales to be made under available statutory exemptions or under a discretionary exemption granted by the applicable Canadian securities regulatory authority. Purchasers are advised to seek legal advice prior to any resale of our common stock.
Representations of Purchasers
By purchasing our common stock in Canada and accepting a purchase confirmation a purchaser is representing to us and the dealer from whom the purchase confirmation is received that:
| | the purchaser is entitled under applicable provincial securities laws to purchase our common stock without the benefit of a prospectus qualified under those securities laws, |
| | where required by law, that the purchaser is purchasing as principal and not as agent, |
| | the purchaser has reviewed the text above under the heading Resale Restrictions, and |
| | the purchaser acknowledges and consents to the provision of specified information concerning its purchase of our common stock to the regulatory authority that by law is entitled to collect the information. |
Further details concerning the legal authority for this information is available on request.
Rights of Action Ontario Purchasers Only
Under Ontario securities legislation, certain purchasers who purchase a security offered by this prospectus during the period of distribution will have a statutory right of action for damages, or while still the owner of the common stock, for rescission against us in the event that this prospectus contains a misrepresentation without regard to whether the purchaser relied on the misrepresentation. The right of action for damages is exercisable not later than the earlier of 180 days from the date the purchaser first had knowledge of the facts giving rise to the cause of action and three years from the date on which payment is made for the common stock. The right of action for rescission is exercisable not later than 180 days from the date on which payment is made for the common stock. If a purchaser elects to exercise the right of action for rescission, the purchaser will have no right of action for damages against us. In no case will the amount recoverable in any action exceed the price at which the common stock was offered to the purchaser and if the purchaser is shown to have purchased the securities with knowledge of the misrepresentation, we will have no liability. In the case of an action for damages, we will not be liable for all or any portion of the damages that are proven to not represent the depreciation in value of the common stock as a result of the misrepresentation relied upon. These rights are in addition to, and without derogation from, any other rights or remedies available at law to an Ontario purchaser. The foregoing is a summary of the rights available to an Ontario purchaser. Ontario purchasers should refer to the complete text of the relevant statutory provisions.
Enforcement of Legal Rights
All of our directors and officers as well as the experts named herein may be located outside of Canada and, as a result, it may not be possible for Canadian purchasers to effect service of process within Canada upon us or those persons. All or a substantial portion of our assets and the assets of those persons may be located outside of Canada and, as a result, it may not be possible to satisfy a judgment against us or those persons in Canada or to enforce a judgment obtained in Canadian courts against us or those persons outside of Canada.
157
Table of Contents
Taxation and Eligibility for Investment
Canadian purchasers of our common stock should consult their own legal and tax advisors with respect to the tax consequences of an investment in our common stock in their particular circumstances and about the eligibility of our common stock for investment by the purchaser under relevant Canadian legislation.
158
Table of Contents
The validity of our common stock offered by this prospectus will be passed upon for us by Latham & Watkins LLP, Menlo Park, California. Certain attorneys and investment funds affiliated with the firm collectively own less than 1% of our shares of preferred stock, which will convert into an aggregate of less than 1% of our shares of common stock upon the completion of this offering. Certain legal matters in connection with this offering will be passed upon for the underwriters by Wilson Sonsini Goodrich & Rosati, Professional Corporation, Palo Alto, California.
The consolidated financial statements of Codexis, Inc. at December 31, 2007 and 2008, and for each of the three years in the period ended December 31, 2008, appearing in this Prospectus and Registration Statement have been audited by Ernst & Young LLP, independent registered public accounting firm, as set forth in their report thereon appearing elsewhere herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the Securities and Exchange Commission a registration statement on Form S-1 under the Securities Act, with respect to the shares of our common stock offered hereby. This prospectus does not contain all of the information set forth in the registration statement and the exhibits and schedules thereto. Some items are omitted in accordance with the rules and regulations of the SEC. For further information with respect to us and the common stock offered hereby, we refer you to the registration statement and the exhibits and schedules filed therewith. Statements contained in this prospectus as to the contents of any contract, agreement or any other document are summaries of the material terms of this contract, agreement or other document. A copy of the registration statement, and the exhibits and schedules thereto, may be inspected without charge at the public reference facilities maintained by the SEC at 100 F Street, N.E., Washington, D.C. 20549. Copies of these materials may be obtained by writing to the Public Reference Section of the SEC at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference facility. The SEC maintains a web site that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC. The address of the SECs website is http://www.sec.gov.
Upon completion of this offering, we will become subject to the information and periodic reporting requirements of the Exchange Act and, in accordance therewith, will file periodic reports, proxy statements and other information with the SEC. Such periodic reports, proxy statements and other information will be available for inspection and copying at the public reference room and web site of the SEC referred to above. We maintain a website at www.codexis.com. You may access our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act with the SEC free of charge at our website as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. The reference to our website address does not constitute incorporation by reference of the information contained on our website.
159
Table of Contents
Index to Consolidated Financial Statements
Contents
| F-2 | ||
| F-3 | ||
| F-4 | ||
| Consolidated Statements of Redeemable Convertible Preferred Stock and Stockholders Deficit |
F-5 | |
| F-7 | ||
| F-8 |
F-1
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
Codexis, Inc.
We have audited the accompanying consolidated balance sheets of Codexis, Inc. (the Company) as of December 31, 2007 and 2008, and the related consolidated statements of operations, redeemable convertible preferred stock and stockholders deficit, and cash flows for each of the three years in the period ended December 31, 2008. These financial statements are the responsibility of the Companys management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Companys internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Companys internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Codexis, Inc. at December 31, 2007 and 2008, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2008, in conformity with U.S. generally accepted accounting principles.
/s/ Ernst & Young LLP
Palo Alto, CA
April 28, 2009
F-2
Table of Contents
Consolidated Balance Sheets
(In Thousands, Except Share and Per Share Amounts)
| December 31, 2007 |
December 31, 2008 |
September 30, 2009 |
Pro Forma as of September 30, 2009 |
|||||||||||||
| (Unaudited) | (Unaudited) | |||||||||||||||
| (Note 2) | ||||||||||||||||
| Assets |
||||||||||||||||
| Current assets: |
||||||||||||||||
| Cash and cash equivalents |
$ | 55,075 | $ | 21,903 | $ | 22,644 | $ | 22,644 | ||||||||
| Marketable securities |
28,995 | 15,227 | 33,318 | 33,318 | ||||||||||||
| Accounts receivable, net of allowances of $250, $16 and $82 at December 31, 2007, 2008 and September 30, 2009 (unaudited), respectively |
4,752 | 6,193 | 6,865 | 6,865 | ||||||||||||
| Related party accounts receivable |
1,680 | | 172 | 172 | ||||||||||||
| Inventories |
1,635 | 2,976 | 2,487 | 2,487 | ||||||||||||
| Prepaid expenses and other current assets |
1,209 | 1,669 | 2,168 | 2,168 | ||||||||||||
| Restricted cash |
1,563 | 366 | 594 | 594 | ||||||||||||
| Total current assets |
94,909 | 48,334 | 68,248 | 68,248 | ||||||||||||
| Restricted cash, non-current portion |
632 | 558 | 136 | 136 | ||||||||||||
| Property and equipment, net |
11,099 | 16,006 | 18,839 | 18,839 | ||||||||||||
| Intangible assets, net |
2,783 | 1,793 | 1,151 | 1,151 | ||||||||||||
| Goodwill |
3,099 | 3,137 | 3,209 | 3,209 | ||||||||||||
| Other non-current assets |
1,019 | 1,054 | 1,624 | 1,624 | ||||||||||||
| Total assets |
$ | 113,541 | $ | 70,882 | $ | 93,207 | $ | 93,207 | ||||||||
| Liabilities, Redeemable Convertible Preferred Stock and Stockholders Equity (Deficit) |
||||||||||||||||
| Current liabilities: |
||||||||||||||||
| Accounts payable |
$ | 4,225 | $ | 9,166 | $ | 8,236 | $ | 8,236 | ||||||||
| Accrued compensation |
3,182 | 4,084 | 5,211 | 5,211 | ||||||||||||
| Related party payable |
7,788 | 435 | 344 | 344 | ||||||||||||
| Other accrued liabilities |
7,480 | 8,557 | 9,134 | 9,134 | ||||||||||||
| Advances from a related party |
| 3,000 | | | ||||||||||||
| Redeemable convertible preferred stock warrant liability |
1,485 | 1,382 | 1,731 | | ||||||||||||
| Deferred revenues |
654 | 771 | 863 | 863 | ||||||||||||
| Related party deferred revenues |
4,856 | 9,812 | 8,575 | 8,575 | ||||||||||||
| Financing obligations |
4,507 | 5,194 | 6,093 | 6,093 | ||||||||||||
| Total current liabilities |
34,177 | 42,401 | 40,187 | 38,456 | ||||||||||||
| Deferred revenues, net of current portion |
2,233 | 2,060 | 1,903 | 1,903 | ||||||||||||
| Related party deferred revenues, net of current portion |
16,632 | 11,572 | 8,508 | 8,508 | ||||||||||||
| Financing obligations, net of current portion |
12,900 | 8,154 | 3,412 | 3,412 | ||||||||||||
| Other long-term liabilities |
2,321 | 3,073 | 2,344 | 2,344 | ||||||||||||
| Commitments and contingencies (Note 9) |
||||||||||||||||
| Redeemable convertible preferred stock issuable in series A to F, $0.0001 par value per share; 33,204,886, 33,204,886 and 39,204,886 shares authorized at December 31, 2007, 2008 and September 30, 2009 (unaudited), respectively; 32,269,494, 32,269,494 and 37,563,610 shares issued and outstanding at December 31, 2007, 2008 and September 30, 2009 (unaudited), respectively; aggregate liquidation value of $204,006 at September 30, 2009 (unaudited); no shares authorized, issued or outstanding pro forma (unaudited) |
132,746 | 132,746 | 177,746 | | ||||||||||||
| Stockholders equity (deficit): |
||||||||||||||||
| Common stock, $0.0001 par value per share; 62,000,000, 62,000,000 and 68,000,000 shares authorized at December 31, 2007, 2008 and September 30, 2009 (unaudited), respectively; 3,386,789, 3,905,425 and 3,975,552 shares issued and outstanding at December 31, 2007, 2008 and September 30, 2009 (unaudited), respectively; 68,000,000 shares authorized, 41,599,768 shares issued and outstanding pro forma (unaudited) |
| | | 4 | ||||||||||||
| Additional paid-in capital |
6,187 | 10,056 | 13,324 | 192,797 | ||||||||||||
| Accumulated other comprehensive income |
537 | 139 | 211 | 211 | ||||||||||||
| Accumulated deficit |
(94,192 | ) | (139,319 | ) | (154,428 | ) | (154,428 | ) | ||||||||
| Total stockholders equity (deficit) |
(87,468 | ) | (129,124 | ) | (140,893 | ) | 38,584 | |||||||||
| Total liabilities, redeemable convertible preferred stock and stockholders equity (deficit) |
$ | 113,541 | $ | 70,882 | $ | 93,207 | $ | 93,207 | ||||||||
F-3
Table of Contents
Consolidated Statements of Operations
(In Thousands, Except Per Share Amounts)
| Years Ended December 31, | Nine Months Ended September 30, |
|||||||||||||||||||
| 2006 | 2007 | 2008 | 2008 | 2009 | ||||||||||||||||
| (Unaudited) | ||||||||||||||||||||
| Revenues: |
||||||||||||||||||||
| Product |
$ | 2,544 | $ | 11,418 | $ | 16,860 | $ | 10,777 | $ | 13,401 | ||||||||||
| Related party collaborative research and development |
863 | 8,481 | 30,239 | 18,174 | 43,963 | |||||||||||||||
| Collaborative research and development |
8,403 | 4,733 | 3,062 | 2,678 | 1,295 | |||||||||||||||
| Government grants |
317 | 701 | 317 | 251 | 11 | |||||||||||||||
| Total revenues |
12,127 | 25,333 | 50,478 | 31,880 | 58,670 | |||||||||||||||
| Costs and operating expenses: |
||||||||||||||||||||
| Cost of product revenues |
1,806 | 8,319 | 13,188 | 7,922 | 11,886 | |||||||||||||||
| Research and development |
17,257 | 35,644 | 45,554 | 33,250 | 39,486 | |||||||||||||||
| Selling, general and administrative |
11,880 | 19,713 | 35,709 | 28,300 | 20,939 | |||||||||||||||
| Total costs and operating expenses |
30,943 | 63,676 | 94,451 | 69,472 | 72,311 | |||||||||||||||
| Loss from operations |
(18,816 | ) | (38,343 | ) | (43,973 | ) | (37,592 | ) | (13,641 | ) | ||||||||||
| Interest income |
742 | 1,491 | 1,538 | 1,435 | 141 | |||||||||||||||
| Interest expense and other, net |
(724 | ) | (2,533 | ) | (2,365 | ) | (2,517 | ) | (1,530 | ) | ||||||||||
| Loss before provision (benefit) for income taxes |
(18,798 | ) | (39,385 | ) | (44,800 | ) | (38,674 | ) | (15,030 | ) | ||||||||||
| Provision (benefit) for income taxes |
(127 | ) | (408 | ) | 327 | 126 | 79 | |||||||||||||
| Net loss |
$ | (18,671 | ) | $ | (38,977 | ) | $ | (45,127 | ) | $ | (38,800 | ) | $ | (15,109 | ) | |||||
| Net loss per share of common stock, basic and diluted |
$ | (10.99 | ) | $ | (15.53 | ) | $ | (12.64 | ) | $ | (11.02 | ) | $ | (3.86 | ) | |||||
| Weighted average common shares used in computing net loss per share of common stock, basic and diluted |
1,699 | 2,510 | 3,570 | 3,521 | 3,917 | |||||||||||||||
| Net loss used in computing pro forma net loss per share of common stock, basic and diluted (unaudited) (Note 2) |
$ | (45,230 | ) | $ | (14,760 | ) | ||||||||||||||
| Pro forma net loss per share of common stock, basic and diluted (unaudited) (Note 2) |
$ | (1.26 | ) | $ | (0.37 | ) | ||||||||||||||
| Weighted average common shares used in computing pro forma net loss per share of common stock, basic and diluted (unaudited) (Note 2) |
35,900 | 39,684 | ||||||||||||||||||
F-4
Table of Contents
Consolidated Statements of Redeemable Convertible Preferred Stock and Stockholders Deficit
(In Thousands)
| Redeemable Convertible Preferred Stock |
Common Stock | Additional Paid-in Capital |
Accumulated Other Compre- hensive Income (Loss) |
Accumulated Deficit |
Total Stockholders Deficit |
||||||||||||||||||||||
| Shares | Amount | Shares | Amount | ||||||||||||||||||||||||
| December 31, 2005 |
15,616 | $ | 37,749 | 1,637 | $ | | $ | 2,352 | $ | (581 | ) | $ | (36,544 | ) | $ | (34,773 | ) | ||||||||||
| Exercise of stock options |
| | 125 | | 55 | | | 55 | |||||||||||||||||||
| Issuance of common stock related to an acquisition |
| | 36 | | 25 | | | 25 | |||||||||||||||||||
| Issuance of Series D redeemable convertible preferred stock, net of issuance costs of $208 |
8,989 | 35,482 | | | | | | | |||||||||||||||||||
| Beneficial conversion feature on issuance of redeemable convertible preferred stock warrants in connection with convertible debt |
| | | | 5 | | | 5 | |||||||||||||||||||
| Issuance of Series D redeemable convertible preferred stock upon conversion of convertible debt and accrued interest |
1,079 | 4,282 | | | | | | | |||||||||||||||||||
| Employee stock-based compensation |
| | | | 32 | | | 32 | |||||||||||||||||||
| Non-employee stock-based compensation |
| | | | 32 | | | 32 | |||||||||||||||||||
| Comprehensive loss: |
|||||||||||||||||||||||||||
| Net loss |
| | | | | | (18,671 | ) | (18,671 | ) | |||||||||||||||||
| Currency translation adjustments |
| | | | | 528 | | 528 | |||||||||||||||||||
| Unrealized gain on marketable securities |
| | | | | 1 | | 1 | |||||||||||||||||||
| Total comprehensive loss |
(18,142 | ) | |||||||||||||||||||||||||
| December 31, 2006 |
25,684 | 77,513 | 1,798 | | 2,501 | (52 | ) | (55,215 | ) | (52,766 | ) | ||||||||||||||||
| Exercise of stock options |
| | 596 | | 265 | | | 265 | |||||||||||||||||||
| Vesting of shares exercised early |
| | | | 38 | | | 38 | |||||||||||||||||||
| Employee stock-based compensation |
| | | | 1,043 | | | 1,043 | |||||||||||||||||||
| Non-employee stock-based compensation |
| | | | 213 | | | 213 | |||||||||||||||||||
| Issuance of common stock related to an acquisition |
| | 963 | | 1,228 | | | 1,228 | |||||||||||||||||||
| Issuance of common stock in connection with a license agreement |
| | 30 | | 134 | | | 134 | |||||||||||||||||||
| Issuance of Series D redeemable convertible preferred stock upon exercise of warrants |
429 | 3,000 | | | 765 | | | 765 | |||||||||||||||||||
| Issuance of Series E redeemable convertible preferred stock, net of issuance costs of $100 |
6,101 | 51,753 | | | | | | | |||||||||||||||||||
| Issuance of Series E redeemable convertible preferred stock for consulting services |
56 | 480 | | | | | | | |||||||||||||||||||
| Comprehensive loss: |
|||||||||||||||||||||||||||
| Net loss |
| | | | | | (38,977 | ) | (38,977 | ) | |||||||||||||||||
| Currency translation adjustments |
| | | | | 457 | | 457 | |||||||||||||||||||
| Unrealized gain on marketable securities |
| | | | | 132 | | 132 | |||||||||||||||||||
| Total comprehensive loss |
(38,388 | ) | |||||||||||||||||||||||||
| December 31, 2007 |
32,270 | 132,746 | 3,387 | | 6,187 | 537 | (94,192 | ) | (87,468 | ) | |||||||||||||||||
| Exercise of stock options |
| | 518 | | 378 | | | 378 | |||||||||||||||||||
| Vesting of shares exercised early |
| | | | 31 | | | 31 | |||||||||||||||||||
| Employee stock-based compensation |
| | | | 3,163 | | | 3,163 | |||||||||||||||||||
| Non-employee stock-based compensation |
| | | | 297 | | | 297 | |||||||||||||||||||
| Comprehensive loss: |
|||||||||||||||||||||||||||
| Net loss |
| | | | | | (45,127 | ) | (45,127 | ) | |||||||||||||||||
| Currency translation adjustments |
| | | | | (278 | ) | | (278 | ) | |||||||||||||||||
| Unrealized loss on marketable securities |
| | | | | (120 | ) | | (120 | ) | |||||||||||||||||
| Total comprehensive loss |
(45,525 | ) | |||||||||||||||||||||||||
| December 31, 2008 |
32,270 | 132,746 | 3,905 | | 10,056 | 139 | (139,319 | ) | (129,124 | ) | |||||||||||||||||
F-5
Table of Contents
Codexis, Inc.
Consolidated Statements of Redeemable Convertible Preferred Stock and Stockholders Deficit (Continued)
(In Thousands)
| Redeemable Convertible Preferred Stock |
Common Stock | Additional Paid-in Capital |
Accumulated Other Compre- hensive Income (Loss) |
Accumulated Deficit |
Total Stockholders Deficit |
|||||||||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||||||||||||
| Exercise of stock options (unaudited) |
| | 71 | | 72 | | | 72 | ||||||||||||||||||
| Vesting of shares exercised early (unaudited) |
| | | | 17 | | | 17 | ||||||||||||||||||
| Employee stock-based compensation (unaudited) |
| | | | 3,067 | | | 3,067 | ||||||||||||||||||
| Non-employee stock-based compensation (unaudited) |
| | | | 112 | | | 112 | ||||||||||||||||||
| Issuance of Series F redeemable convertible preferred stock (unaudited) |
5,294 | 45,000 | | | | | | | ||||||||||||||||||
| Comprehensive loss: |
||||||||||||||||||||||||||
| Net loss (unaudited) |
| | | | | | (15,109 | ) | (15,109 | ) | ||||||||||||||||
| Currency translation adjustments (unaudited) |
| | | | | 58 | | 58 | ||||||||||||||||||
| Unrealized gain on marketable securities (unaudited) |
| | | | | 14 | | 14 | ||||||||||||||||||
| Total comprehensive loss (unaudited) |
(15,037 | ) | ||||||||||||||||||||||||
| September 30, 2009 (unaudited) |
37,564 | $ | 177,746 | 3,976 | $ | | $ | 13,324 | $ | 211 | $ | (154,428 | ) | $ | (140,893 | ) | ||||||||||
F-6
Table of Contents
Consolidated Statements of Cash Flows
(In Thousands)
| Years Ended December 31, | Nine Months Ended September 30, |
|||||||||||||||||||
| 2006 | 2007 | 2008 | 2008 | 2009 | ||||||||||||||||
| (Unaudited) | ||||||||||||||||||||
| Operating activities |
||||||||||||||||||||
| Net loss |
$ | (18,671 | ) | $ | (38,977 | ) | $ | (45,127 | ) | $ | (38,800 | ) | $ | (15,109 | ) | |||||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||||||||||
| Amortization of intangible assets |
633 | 781 | 880 | 784 | 709 | |||||||||||||||
| Depreciation and amortization of property and equipment |
1,754 | 2,103 | 3,683 | 2,565 | 3,722 | |||||||||||||||
| Revaluation of redeemable convertible preferred stock warrant liability |
156 | 1,328 | (103 | ) | 567 | 349 | ||||||||||||||
| Loss (gain) on disposal of property and equipment |
6 | 86 | 2 | 1 | (50 | ) | ||||||||||||||
| Stock-based compensation |
64 | 1,256 | 3,460 | 2,376 | 3,179 | |||||||||||||||
| Amortization of debt discount |
5 | 67 | 531 | 403 | 283 | |||||||||||||||
| Accretion (amortization) of premium/discount on marketable securities |
| (368 | ) | (676 | ) | (657 | ) | 368 | ||||||||||||
| Amortization of deferred costs associated with a license agreement |
62 | 400 | | | | |||||||||||||||
| Beneficial conversion feature on issuance of redeemable convertible preferred stock |
5 | | | | | |||||||||||||||
| Issuance of redeemable convertible preferred stock for consulting services |
| 480 | | | | |||||||||||||||
| Issuance of common stock in connection with a license agreement |
| 134 | | | | |||||||||||||||
| Changes in operating assets and liabilities: |
||||||||||||||||||||
| Accounts receivable |
369 | (3,146 | ) | 226 | 1,040 | (842 | ) | |||||||||||||
| Inventories |
(354 | ) | (283 | ) | (1,382 | ) | (1,431 | ) | 511 | |||||||||||
| Prepaid expenses and other current assets |
(211 | ) | (285 | ) | (460 | ) | (1,618 | ) | (499 | ) | ||||||||||
| Other assets |
(191 | ) | (590 | ) | (113 | ) | (593 | ) | (629 | ) | ||||||||||
| Accounts payable |
1,409 | 1,169 | 4,941 | 1,723 | (929 | ) | ||||||||||||||
| Accrued compensation |
552 | 1,664 | 902 | 1,348 | 1,128 | |||||||||||||||
| Related party payable |
560 | 7,228 | (7,353 | ) | (7,506 | ) | (91 | ) | ||||||||||||
| Deferred revenues |
775 | 16,385 | (160 | ) | (1,050 | ) | (4,366 | ) | ||||||||||||
| Other accrued liabilities |
(210 | ) | 4,098 | 4,433 | 2,231 | (3,266 | ) | |||||||||||||
| Net cash used in operating activities |
(13,287 | ) | (6,470 | ) | (36,316 | ) | (38,617 | ) | (15,532 | ) | ||||||||||
| Investing activities |
||||||||||||||||||||
| Decrease (increase) in restricted cash |
(193 | ) | (1,301 | ) | 1,271 | 1,271 | 194 | |||||||||||||
| Purchase of property and equipment |
(1,102 | ) | (8,245 | ) | (8,537 | ) | (5,587 | ) | (6,491 | ) | ||||||||||
| Purchase of marketable securities |
| (42,267 | ) | (47,821 | ) | (37,727 | ) | (35,619 | ) | |||||||||||
| Proceeds from maturities of marketable securities |
1,500 | 13,772 | 56,062 | 39,084 | 17,175 | |||||||||||||||
| Proceeds from sale of marketable securities |
| | 6,081 | 1,247 | | |||||||||||||||
| Acquisition, net of cash acquired |
| (1,168 | ) | | | | ||||||||||||||
| Net cash provided by (used in) investing activities |
205 | (39,209 | ) | 7,056 | (1,712 | ) | (24,741 | ) | ||||||||||||
| Financing activities |
||||||||||||||||||||
| Proceeds from financing obligations |
1,067 | 14,805 | | | | |||||||||||||||
| Principal payments on financing obligations |
(1,090 | ) | (1,485 | ) | (4,264 | ) | (2,919 | ) | (4,004 | ) | ||||||||||
| Proceeds from convertible debt |
4,200 | | | | | |||||||||||||||
| Proceeds from the exercise of redeemable convertible preferred stock warrants |
| 3,000 | | | | |||||||||||||||
| Proceeds from issuance of preferred stock, net of issuance costs |
35,482 | 51,753 | | | 45,000 | |||||||||||||||
| Proceeds from exercises of stock options |
55 | 303 | 378 | 265 | 72 | |||||||||||||||
| Net cash provided by (used in) financing activities |
39,714 | 68,376 | (3,886 | ) | (2,654 | ) | 41,068 | |||||||||||||
| Effect of exchange rate changes on cash and cash equivalents |
109 | 132 | (26 | ) | (81 | ) | (54 | ) | ||||||||||||
| Net increase (decrease) in cash and cash equivalents |
26,741 | 22,829 | (33,172 | ) | (43,064 | ) | 741 | |||||||||||||
| Cash and cash equivalents at beginning of period |
5,505 | 32,246 | 55,075 | 55,075 | 21,903 | |||||||||||||||
| Cash and cash equivalents at end of period |
$ | 32,246 | $ | 55,075 | $ | 21,903 | $ | 12,011 | $ | 22,644 | ||||||||||
| Supplemental disclosures of cash flow information: |
||||||||||||||||||||
| Cash paid for interest |
$ | 383 | $ | 686 | $ | 1,572 | $ | 1,421 | $ | 964 | ||||||||||
| Cash paid for income taxes |
$ | 132 | $ | 99 | $ | 80 | $ | 80 | $ | 110 | ||||||||||
| Supplemental schedule of noncash investing and financing activities: |
||||||||||||||||||||
| Conversion of convertible debt to redeemable convertible preferred stock |
$ | 4,282 | $ | | $ | | $ | | $ | | ||||||||||
| Issuance of redeemable convertible preferred stock warrants in connection with financing arrangement |
$ | 736 | $ | 463 | $ | | $ | | $ | | ||||||||||
| Issuance of common stock for acquisitions |
$ | 25 | $ | 1,228 | $ | | $ | | $ | | ||||||||||
F-7
Table of Contents
Notes to Consolidated Financial Statements
1. Description of Business
Codexis, Inc. (we or Codexis) is a developer of proprietary biocatalysts that we believe have the potential to make existing industrial processes faster, cleaner and more efficient than current methods and have the potential to make new industrial processes possible on a commercial scale. Biocatalysts are enzymes or microbes that initiate or accelerate chemical reactions. We are currently selling our biocatalysts to customers in the pharmaceutical industry and are engaged in a multi-year research and development collaboration with Equilon Enterprises LLC dba Shell Oil Products US (Shell) to develop advanced biofuels. We are also using our technology platform to pursue biocatalyst-enabled solutions in other bioindustrial markets, including carbon management, water treatment and chemicals. We were incorporated in Delaware in January 2002.
2. Summary of Significant Accounting Policies
Basis of Presentation and Consolidation
The consolidated financial statements have been prepared in conformity with generally accepted accounting principles in the United States and include the accounts of Codexis and our wholly-owned subsidiaries. The results of operations of BioCatalytics, Inc., a California corporation (BioCatalytics), are included in the accompanying consolidated statements of operations subsequent to its acquisition on July 17, 2007. We also have subsidiaries in Germany, Singapore, India, Austria, Mauritius, and Hungary. All significant intercompany balances and transactions have been eliminated in consolidation.
Redeemable Convertible Preferred Stock
The holders of at least a majority of the then-outstanding shares of Series B, D and E redeemable convertible preferred stock, voting or consenting as separate series, may require us to redeem each of the respective series of redeemable convertible preferred stock on or after December 31, 2013. The holders of Series A, C and F convertible preferred stock do not have redemption rights; however, the securities are classified outside of stockholders deficit due to their liquidation rights. The holders of our Series A, B, C, D, E and F preferred stock control the vote of our stockholders and board of directors through their appointed representatives. As a result, the holders of Series A, B, C, D, E and F preferred stock can force a change in control that would trigger liquidation. As redemption of the preferred stock through liquidation is outside of our control, all shares of preferred stock have been presented outside of permanent equity on our consolidated balance sheets. Series A, B, C, D, E and F preferred stock are collectively referred to in the consolidated financial statements and notes to the consolidated financial statements as redeemable convertible preferred stock.
Unaudited Interim Financial Information
The accompanying consolidated balance sheet as of September 30, 2009, the consolidated statements of operations and cash flows for the nine months ended September 30, 2008 and 2009, and the consolidated statement of redeemable convertible preferred stock and stockholders deficit for the nine months ended September 30, 2009 are unaudited. The unaudited interim financial statements have been prepared on the same basis as the annual consolidated financial statements and, in the opinion of management, reflect all adjustments, which include only normal recurring adjustments, necessary to state fairly our financial position as of September 30, 2009 and results of operations and cash flows for the nine months ended September 30, 2008 and 2009. The financial data and other information disclosed in these notes to the financial statements as of September 30, 2009 and for the nine months ended September 30, 2008 and 2009 are unaudited. The results for the nine months ended September 30, 2009 are not necessarily indicative of the results to be expected for the year ending December 31, 2009 or for any future year or interim period.
F-8
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
Unaudited Pro Forma Balance Sheet
In the event that an initial public offering is consummated that results in the automatic conversion of our redeemable convertible preferred stock, as described in Note 11, all of the redeemable convertible preferred stock outstanding will automatically convert into 37,624,216 shares of common stock based on the number of shares of redeemable convertible preferred stock outstanding at September 30, 2009. In addition, all redeemable convertible preferred stock warrants will automatically convert to common stock warrants and the related redeemable convertible preferred stock warrant liability of $1.7 million at September 30, 2009 would be reclassified to additional paid-in capital. The unaudited pro forma balance sheet information at September 30, 2009 gives effect to the automatic conversion of all outstanding shares of the redeemable convertible preferred stock to common stock and the conversion of all redeemable convertible preferred stock warrants to common stock warrants.
Significant Risks and Uncertainties
We have incurred net losses of $18.7 million, $39.0 million, $45.1 million, $38.8 million and $15.1 million for the years ended December 31, 2006, 2007 and 2008 and the nine months ended September 30, 2008 and 2009, respectively. We used $13.3 million, $6.5 million, $36.3 million, $38.6 million and $15.5 million of cash in operating activities for the years ended December 31, 2006, 2007 and 2008 and the nine months ended September 30, 2008 and 2009, respectively. At December 31, 2008 and September 30, 2009, we had an accumulated deficit of $139.3 million and $154.4 million, respectively, and unrestricted cash and cash equivalents and marketable securities of $37.1 million and $56.0 million, respectively. Our failure to generate sufficient revenues, achieve planned gross margins, control operating costs or raise sufficient additional funds may require us to modify, delay or abandon some of our planned future expansion or expenditures, which could have a material adverse effect on our business, operating results, financial condition and ability to achieve our intended business objectives. We may be required to seek additional funds through collaborations or public or private debt or equity financings, and may also seek to reduce expenses related to our operations. There can be no assurance that any financings will be available or at terms acceptable to us.
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosures of contingent liabilities at the date of the consolidated financial statements, and the reported amounts of revenues and expenses during the reporting period. Our management regularly assesses these estimates which primarily affect revenue recognition, the valuation of accounts receivable, intangible assets and goodwill arising out of business acquisitions, inventories, accrued liabilities, the fair values of redeemable convertible preferred stock, common stock, redeemable convertible preferred stock warrants and stock options and the valuation allowances associated with deferred tax assets. Actual results could differ from those estimates, and such differences may be material to the consolidated financial statements.
Foreign Currency Translation
The assets and liabilities of foreign subsidiaries, where the local currency is the functional currency, are translated from their respective functional currencies into U.S. dollars at the exchange rates in effect at the balance sheet date, with resulting foreign currency translation adjustments recorded in accumulated other comprehensive income (loss) in the consolidated statements of stockholders deficit. Revenues and
F-9
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
expense amounts are translated at average rates during the period. Accumulated other comprehensive income (loss) included cumulative translation adjustment losses of $580,000 and $52,000, and gains of $405,000, $127,000 and $185,000 at December 31, 2005, 2006, 2007 and 2008 and September 30, 2009, respectively.
Where the U.S. dollar is the functional currency, nonmonetary assets and liabilities originally acquired or assumed in other currencies are recorded in U.S. dollars at the exchange rates in effect at the date they were acquired or assumed. Monetary assets and liabilities denominated in other currencies are translated into U.S. dollars at the exchange rates in effect at the balance sheet date. Revenues and expense amounts are generally translated at the average rates during the period. Translation adjustments are recorded in interest expense and other, net in the accompanying consolidated statements of operations. Gains and losses realized from transactions, including intercompany balances not considered as permanent investments, denominated in currencies other than an entitys functional currency, are included in interest expense and other, net in the accompanying consolidated statements of operations.
Concentrations of Credit Risk
Financial instruments that potentially subject us to significant concentrations of credit risk consist primarily of cash and cash equivalents, marketable securities, accounts receivable and restricted cash. Cash and cash equivalents, marketable securities and restricted cash are invested through banks and other financial institutions in the United States, as well as in other foreign countries. Such deposits are primarily in excess of insured limits.
Credit risk with respect to accounts receivable exists to the full extent of amounts presented in the consolidated financial statements. We periodically require collateral to support credit sales. We estimate an allowance for doubtful accounts through specific identification of potentially uncollectible accounts receivable based on an analysis of our accounts receivable aging. Uncollectible accounts receivable are written off against the allowance for doubtful accounts when all efforts to collect them have been exhausted. Recoveries are recognized when they are received. Actual collection losses may differ from our estimates and could be material to the consolidated financial position, results of operations, and cash flows.
One customer accounted for 14%, 21% and 39% of accounts receivable at December 31, 2007, December 31, 2008 and September 30, 2009, respectively. At December 31, 2007 and 2008 and September 30, 2009, a second customer accounted for 2%, 37% and 17% of accounts receivable, respectively. One additional customer accounted for 11% of accounts receivable at December 31, 2008. Two additional customers accounted for 11% and 10% of accounts receivable at September 30, 2009. We do not believe the accounts receivable from these customers represent a significant credit risk based on past collection experiences and the general creditworthiness of these customers.
Fair Value of Financial Instruments
The carrying amounts of certain of our financial instruments, including cash and cash equivalents, marketable securities, restricted cash, accounts receivable and accounts payable, approximate fair value due to their short maturities. Based on borrowing rates currently available to us for loans with similar terms, the carrying values of our financing obligations approximate their fair values.
Fair value is considered to be the price at which an asset could be exchanged or a liability transferred (an exit price) in an orderly transaction between knowledgeable, willing parties in the principal or most advantageous market for the asset or liability. Where available, fair value is based on or derived from observable market prices or other observable inputs. Where observable prices or inputs are not available,
F-10
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
valuation models are applied. These valuation techniques involve some level of management estimation and judgment, the degree of which is dependent on the price transparency for the instruments or market and the instruments complexity.
Cash, Cash Equivalents and Marketable Securities
We consider all highly liquid investments with maturity dates of three months or less at the date of purchase to be cash equivalents. Cash and cash equivalents consist of cash on deposit with banks and money market funds. Marketable securities are primarily comprised of corporate debt obligations, U.S. Treasury obligations and government-sponsored enterprise securities.
Our debt securities are classified as available-for-sale and are carried at estimated fair value. Unrealized gains and losses are reported as a component of accumulated other comprehensive income (loss). Amortization of purchase premiums and accretion of purchase discounts, realized gains and losses and declines in value deemed to be other than temporary, if any, are included in interest income or interest expense and other, net. The cost of securities sold is based on the specific-identification method. There were no significant realized gains or losses from sales of marketable securities during the years ended December 31, 2006, 2007 and 2008 or the nine months ended September 30, 2009. At December 31, 2007, December 31, 2008 and September 30, 2009, we did not have any other-than-temporary declines in the fair value of our marketable securities.
Accounts Receivable
Accounts receivable represent amounts owed to us under our collaborative research and development agreements, product sales, and government grants. Our allowance for doubtful accounts was $250,000, $16,000 and $82,000 as of December 31, 2007, December 31, 2008 and September 30, 2009, respectively. Specific accounts written off against the established reserve were $141,000, $0, $234,000, and $0 during the years ended December 31, 2006, 2007 and 2008 and the nine months ended September 30, 2009, respectively.
Inventories
Inventories consist of biocatalysts, which are enzymes or microbes that facilitate chemical reactions, and pharmaceutical intermediates. Inventories are held in our facilities in the United States and Europe and at contract manufacturers in Europe and Asia. Internally produced biocatalysts only qualify as commercial inventory after they have achieved specifications that are required for selling the materials. Inventories held at our contract manufacturers are accepted as finished goods after achieving specifications stated in our purchase orders. Inventories are carried at the lower of cost or market and are removed from inventory using the first-in first-out method. Inventories, based on demand and age, are written down for excess and obsolete materials, if necessary.
F-11
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
Property and Equipment
Property and equipment, including the cost of purchased software, are stated at cost, less accumulated depreciation and amortization. Depreciation is calculated using the straight-line method over the following estimated ranges of useful lives:
| Asset classification |
Estimated useful life |
|
| Laboratory equipment |
5 years | |
| Computer equipment and software |
3 to 5 years | |
| Office equipment and furniture |
5 years | |
| Leasehold improvements |
Lesser of useful life or lease term |
Goodwill
Goodwill represents the excess of the purchase price over the fair value of assets acquired and liabilities assumed. Goodwill is presumed to have an indefinite life and is not subject to annual amortization. We review goodwill for impairment at the company level, which is the sole reporting unit, on at least an annual basis and at any interim date whenever events or changes in circumstances indicate that the carrying value may not be recoverable.
The annual test for goodwill impairment is a two-step process. The first step is a comparison of the fair value of the reporting unit with its carrying amount, including goodwill. If this step indicates an impairment, then the loss is measured as the excess of recorded goodwill over its implied fair value. Implied fair value is the excess of the fair value of the reporting unit over the fair value of all identified assets and liabilities. No impairment charges were recorded during the years ended December 31, 2006, 2007 and 2008 or the nine months ended September 30, 2009.
Intangible Assets and Impairment of Long-Lived Assets
Intangible assets consist of customer relationships, developed core technology and the trade name, all arising out of the Jülich Fine Chemicals (JFC) acquisition in 2005 and BioCatalytics acquisition in 2007. Intangible assets are recorded at their fair value at the date of the acquisition and, for those assets having finite useful lives, are amortized using the straight-line method over their estimated useful lives, which range from one to seven years.
We periodically review our intangible and other long-lived assets for possible impairment, whenever events or changes in circumstances indicate that such assets are impaired or the estimated useful lives are no longer appropriate. If indicators of impairment exist and the undiscounted projected cash flows associated with such assets are less than the carrying amounts of the assets, an impairment loss is recorded to write the assets down to their estimated fair values. Fair value is estimated based on discounted future cash flows. No impairment charges were recorded during the years ended December 31, 2006, 2007 and 2008 or the nine months ended September 30, 2008 and 2009.
Restricted Cash
Restricted cash was invested in money market accounts primarily for purposes of securing a standby letter of credit as collateral for our Redwood City, California facility lease agreement, for future payment obligations to the shareholder of BioCatalytics related to the acquisition, and for the purpose of securing a working capital line of credit. During the year ended December 31, 2008, restricted cash decreased by $0.8 million on payment of purchase consideration to a former shareholder of BioCatalytics and $0.6 million on expiration of JFC-related letters of credit relating to its facility lease.
F-12
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
Redeemable Convertible Preferred Stock Warrant Liability
Outstanding warrants to purchase shares of our Series D redeemable convertible preferred stock are freestanding warrants that are subject to redemption and are therefore classified as liabilities on the consolidated balance sheet at fair value. The initial liability recorded is adjusted for changes in fair value at each reporting date with an offsetting entry recorded as a component of interest expense and other, net in the accompanying consolidated statements of operations. The liability will continue to be adjusted for changes in fair value until the earlier of the exercise date or the conversion of the underlying redeemable convertible preferred stock into common stock, at which time the redeemable convertible preferred stock warrants will convert to common stock warrants and the liability will be reclassified to stockholders equity.
Revenue Recognition
When evaluating multiple element arrangements, we consider whether the components of each arrangement represent separate units of accounting. Revenue arrangements with multiple components are divided into separate units of accounting if certain criteria are met, including whether the delivered component has stand-alone value to the customer, and whether there is objective and reliable evidence of the fair value of the undelivered items. Consideration received is allocated among the separate units of accounting based on their respective fair values. Applicable revenue recognition criteria are then applied to each of the units.
Revenues are recognized when the four basic revenue recognition criteria are met: (1) persuasive evidence of an arrangement exists; (2) products have been delivered, transfer of technology has been completed or services have been rendered; (3) the fee is fixed or determinable; and (4) collectability is reasonably assured.
Our primary sources of revenues consist of collaborative research and development agreements, product revenues and government grants. Collaborative research and development agreements typically provide us with multiple revenue streams, including up-front fees for licensing, exclusivity and technology access, fees for full-time employee equivalent (FTE) services and the potential to earn milestone payments upon achievement of contractual criteria and royalty fees based on future product sales or cost savings by our customers. Our collaborative research and development revenues consist of revenues from related parties and revenues from other collaborative research and development agreements. Related party collaborative research and development revenues for the years ended December 31, 2006, 2007 and 2008 and the nine months ended September 30, 2008 and 2009 is comprised of revenue from Shell.
Related party collaborative research and development revenues relate to the arrangements with Shell and consisted of the following (in thousands):
| Years Ended December 31, | Nine Months Ended September 30, |
||||||||||||||
| 2006 | 2007 | 2008 | 2008 | 2009 | |||||||||||
| (Unaudited) | |||||||||||||||
| License, technology access and exclusivity fees |
$ | 373 | $ | 2,665 | $ | 3,675 | $ | 2,236 | $ | 3,500 | |||||
| Services |
365 | 4,909 | 26,564 | 15,938 | 39,963 | ||||||||||
| Milestones |
125 | 907 | | | 500 | ||||||||||
| Total related party collaborative research and development revenues |
$ | 863 | $ | 8,481 | $ | 30,239 | $ | 18,174 | $ | 43,963 | |||||
F-13
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
Other collaborative research and development revenues consisted of the following (in thousands):
| Years Ended December 31, | Nine Months Ended September 30, |
||||||||||||||
| 2006 | 2007 | 2008 | 2008 | 2009 | |||||||||||
| (Unaudited) | |||||||||||||||
| License, technology access and exclusivity fees |
$ | 894 | $ | 1,340 | $ | 150 | $ | 150 | $ | 139 | |||||
| Services |
6,084 | 2,584 | 2,002 | 1,827 | 679 | ||||||||||
| Milestones |
724 | 300 | | | | ||||||||||
| Royalties |
701 | 509 | 910 | 701 | 477 | ||||||||||
| Total collaborative research and development revenues |
$ | 8,403 | $ | 4,733 | $ | 3,062 | $ | 2,678 | $ | 1,295 | |||||
For each source of collaborative research and development revenues, product revenues and grant revenues, we apply the revenue recognition criteria as follows:
| | Up-front fees received in connection with collaborative research and development agreements, including license fees, technology access fees, and exclusivity fees, are deferred upon receipt, are not considered a separate unit of accounting and are recognized as revenues over the relevant performance periods under the agreement, as discussed below. |
| | Revenues related to FTE services are recognized as research services are performed over the related performance periods for each contract. We are required to perform research and development activities as specified in each respective agreement. The payments received are not refundable and are based on a contractual reimbursement rate per FTE working on the project. When up-front payments are combined with FTE services in a single unit of accounting, we recognize the up-front payments using the proportionate performance method of revenue recognition based upon the actual amount of research and development labor hours incurred relative to the amount of the total expected labor hours to be incurred by us, up to the amount of cash received. In cases where the planned levels of research services fluctuate substantially over the research term, we are required to make estimates of the total hours required to perform our obligations. Research and development expenses related to FTE services under the collaborative research and development agreements approximate the research funding over the term of the respective agreements. |
| | Revenues related to milestones that are determined to be substantive and at risk at the inception of the arrangement are recognized upon achievement of the milestone event and when collectability is reasonably assured. Milestone payments are triggered either by the results of our research efforts or by events external to us, such as our collaboration partner achieving a revenue target. Fees associated with milestones for which performance was not at risk at the inception of the arrangement or that are determined not to be substantive are accounted for in the same manner as the up-front fees, provided collectability is reasonably assured. |
| | We recognize revenues from royalties based on licensees sales of products using our technologies. Royalties are recognized as earned in accordance with the contract terms when royalties from licensees can be reasonably estimated and collectability is reasonably assured. |
| | Product revenues are recognized once passage of title and risk of loss has occurred and contractually specified acceptance criteria have been met, provided all other revenue recognition criteria have also been met. Product revenues consist of sales of biocatalysts, intermediates, active pharmaceutical ingredients and Codex Biocatalyst Panels. Cost of product revenues includes both internal and third party fixed and variable costs including amortization of purchased technology, materials and supplies, labor, facilities and other overhead costs associated with our product revenues. |
F-14
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
| | We license mutually agreed upon third party technology for use in our research and development collaboration with Shell. We record the license payments to research and development expense and offset related reimbursements received from Shell. Payments made by Shell to us are direct reimbursements of our costs. We account for these direct reimbursable costs as a net amount, whereby no expense or revenues is recorded for the costs reimbursed by Shell. For any payments not reimbursed by Shell, we will recognize these as expenses in the statement of operations. We elected to present the reimbursement from Shell as a component of our research and development expense since presenting the receipt of payment from Shell as revenues does not reflect the substance of the arrangement. |
| | We receive payments from government entities in the form of government grants. Government grants are agreements that generally provide us with cost reimbursement for certain types of expenditures in return for research and development activities over a contractually defined period. Revenues from government grants are recognized in the period during which the related costs are incurred, provided that the conditions under which the government grants were provided have been met and we have only perfunctory obligations outstanding. |
| | Shipping and handling costs charged to customers are recorded as revenues. Shipping costs are included in our cost of product revenues. Such charges were not significant in any of the periods presented. |
Customer Concentration
Customers with revenues of 10% or more of our total revenues consist of the following (substantially all of the revenues presented below represent revenues from collaborative research and development arrangements):
| Percentage of Total Revenues | |||||||||||||||
| For The Years Ended December 31, |
For The Nine Months Ended September 30, |
||||||||||||||
| 2006 | 2007 | 2008 | 2008 | 2009 | |||||||||||
| (Unaudited) | |||||||||||||||
| Customers |
|||||||||||||||
| Shell |
* | 33 | % | 60 | % | 57 | % | 75 | % | ||||||
| Pfizer |
36 | % | 13 | % | * | * | * | ||||||||
| Schering-Plough |
11 | % | * | * | * | * | |||||||||
| * | Represents less than 10% of total revenues |
Concentrations of Supply Risk
We rely on a limited number of suppliers for our products. We believe that other vendors would be able to provide similar products; however, the qualification of such vendors may require substantial start-up time. In order to mitigate any adverse impacts from a disruption of supply, we attempt to maintain an adequate supply of critical single-sourced materials. For certain materials, our vendors maintain a supply for us. We outsource a portion of the manufacturing of our products to contract manufacturers with facilities in Austria, Germany, India and Italy.
Research and Development Expenses
Research and development expenses consist of costs incurred for internal projects as well as partner-funded collaborative research and development activities. These costs include direct and research-related
F-15
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
overhead expenses, which include salaries, stock-based compensation and other personnel-related expenses, facility costs, supplies, depreciation of facilities, and laboratory equipment, as well as research consultants and the cost of funding research at universities and other research institutions, and are expensed as incurred. Costs to acquire technologies that are utilized in research and development and that have no alternative future use are expensed when incurred.
To approximate research and development expenses by funded category, we estimate, based on FTE efforts, the percentage of research and development efforts, as measured in hours incurred. The number of hours expended in each category is divided by the total number of hours expended on all categories of research and development with the resulting fractions then multiplied by the total cost of research and development effort, with the products then added to project-specific external costs. In the case where a collaborative partner is sharing the research and development costs, the expenses for that project are allocated proportionately between the collaborative projects funded by third parties and internal projects. We believe that presenting our research and development expenses in these categories will provide our investors with meaningful information on how our resources are being used.
The following table presents our approximate research and development expenses by funding category (in thousands):
| Years Ended December 31, |
Nine Months Ended September 30, |
||||||||||||||
| 2006 | 2007 | 2008 | 2008 | 2009 | |||||||||||
| (Unaudited) | |||||||||||||||
| Collaborative research and development(1) |
$ | 4,150 | $ | 10,920 | $ | 17,368 | $ | 10,894 | $ | 28,554 | |||||
| Grants |
25 | 384 | 155 | 113 | | ||||||||||
| Internal projects |
13,082 | 24,340 | 28,031 | 22,243 | 10,932 | ||||||||||
| Total research and development expenses |
$ | 17,257 | $ | 35,644 | $ | 45,554 | $ | 33,250 | $ | 39,486 | |||||
| (1) | Research and development expenses related to collaborative projects funded by related and other third parties are less than the reported revenues due to the recognition of revenues from the amortization of non-refundable up-front payments. |
Advertising
Advertising costs are expensed as incurred and included in selling, general and administrative expenses in the consolidated statements of operations. Advertising costs were $191,000, $244,000 and $335,000 for the years ended December 31, 2006, 2007 and 2008, respectively.
Income Taxes
We use the asset and liability method of accounting for income taxes. Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the consolidated financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets are recognized for deductible temporary differences, along with net operating loss carryforwards, if it is more likely than not that the tax benefits will be realized. To the extent a deferred tax asset cannot be recognized under the preceding criteria, a valuation allowance is established. Deferred tax assets and liabilities are measured using enacted tax rates in effect for the year in which those temporary differences are expected to be recovered or settled.
F-16
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
We adopted FASB Interpretation No. 48, Accounting for Uncertainties in Income Taxes an interpretation of FASB Statement No. 109, or FIN 48 (incorporated in Accounting Standards Codification (ASC) Topic 740, Income Taxes), effective January 1, 2007. FIN 48 requires us to recognize the financial statement effects of a tax position when it is more likely than not, based on the technical merits, that the position will be sustained upon examination. Upon adoption of FIN 48, there was no adjustment to accumulated deficit.
Stock-Based Compensation
Effective January 1, 2006, we began recognizing compensation expense related to share-based transactions, including the awarding of employee stock options, based on the estimated fair value of the awards granted. Options granted prior to January 1, 2006 were measured using the minimum value method for the pro forma disclosures that were previously required. We continued to account for non-vested employee share-based awards outstanding at January 1, 2006 using the intrinsic value method. All awards granted, modified or settled after January 1, 2006 have been accounted for based on the fair value of the awards granted. We are using the straight-line method to allocate stock-based compensation expense to the appropriate reporting periods.
We account for stock options issued to non-employees based on their estimated fair value determined using the Black-Scholes option-pricing model. The fair value of the options granted to non-employees is remeasured as they vest, and the resulting change in value, if any, is recognized as an increase or decrease in stock compensation expense during the period the related services are rendered.
Comprehensive Loss
We report our comprehensive loss, and its components, on the consolidated statements of stockholders deficit. Comprehensive loss consists of net loss, unrealized gains (losses) on marketable securities and foreign currency translation adjustments.
Net Loss per Share of Common Stock
Basic net loss per share of common stock is computed by dividing the net loss by the weighted-average number of shares of common stock outstanding during the period, less the weighted-average unvested common stock subject to repurchase. Diluted net loss per share of common stock is computed by giving effect to all potential common shares, consisting of stock options, warrants and redeemable convertible preferred stock, to the extent dilutive. Basic and diluted net loss per share of common stock was the same for each period presented as the inclusion of all potential common shares outstanding was anti-dilutive.
The calculations for the unaudited pro forma basic and diluted net loss per share of common stock assume the conversion of all outstanding shares of redeemable convertible preferred stock into shares of common stock and the conversion of redeemable convertible preferred stock warrants to common stock warrants as if the conversions had occurred at the beginning of the period, or for Series F redeemable convertible preferred stock issued during the nine months ended September 30, 2009, the issue date for each share, using the as-if-converted method. Also, the numerator in the pro forma basic and diluted net loss per share calculation has been adjusted to remove gains and losses resulting from re-measurements of the redeemable convertible preferred stock warrant liability as these measurements would no longer be required when the redeemable convertible preferred stock warrants become warrants to purchase shares of our common stock.
F-17
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
The following table presents the calculation of historical and pro forma basic and diluted net loss per share of common stock (in thousands, except per share amounts):
| Years Ended December 31, | Nine Months Ended September 30, |
|||||||||||||||||||
| 2006 | 2007 | 2008 | 2008 | 2009 | ||||||||||||||||
| (Unaudited) | ||||||||||||||||||||
| Actual: |
||||||||||||||||||||
| Numerator: |
||||||||||||||||||||
| Net loss |
$ | (18,671 | ) | $ | (38,977 | ) | $ | (45,127 | ) | $ | (38,800 | ) | $ | (15,109 | ) | |||||
| Denominator: |
||||||||||||||||||||
| Weighted-average shares of common stock outstanding |
1,699 | 2,530 | 3,607 | 3,559 | 3,935 | |||||||||||||||
| Less: Weighted-average shares of common stock subject to repurchase |
| (20 | ) | (37 | ) | (38 | ) | (18 | ) | |||||||||||
| Weighted-average shares of common stock used in computing net loss per share of common stock, basic and diluted |
1,699 | 2,510 | 3,570 | 3,521 | 3,917 | |||||||||||||||
| Net loss per share of common stock, basic and diluted |
$ | (10.99 | ) | $ | (15.53 | ) | $ | (12.64 | ) | $ | (11.02 | ) | $ | (3.86 | ) | |||||
| Pro Forma: |
||||||||||||||||||||
| Numerator: |
||||||||||||||||||||
| Net loss |
$ | (45,127 | ) | $ | (15,109 | ) | ||||||||||||||
| Less: change in fair value of redeemable convertible preferred stock warrant liability (unaudited) |
(103 | ) | 349 | |||||||||||||||||
| Net loss used in computing pro forma net loss per share of common stock, basic and diluted (unaudited) |
$ | (45,230 | ) | $ | (14,760 | ) | ||||||||||||||
| Denominator: |
||||||||||||||||||||
| Weighted-average shares of common stock used in computing net loss per share of common stock, basic and diluted, as used above |
3,570 | 3,917 | ||||||||||||||||||
| Add: Pro forma adjustments to reflect weighted-average effect of assumed conversion of redeemable convertible preferred stock (unaudited) |
32,330 | 35,767 | ||||||||||||||||||
| Weighted-average shares of common stock used in computing pro forma net loss per share of common stock, basic and diluted (unaudited) |
35,900 | 39,684 | ||||||||||||||||||
| Pro forma net loss per share of common stock, basic and diluted (unaudited) |
$ | (1.26 | ) | $ | (0.37 | ) | ||||||||||||||
F-18
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
The following redeemable convertible preferred stock, common stock subject to repurchase, options to purchase common stock, warrants to purchase redeemable convertible preferred and warrants to purchase common stock were excluded from the computation of diluted net loss per share of common stock for the periods presented because including them would have had an antidilutive effect (in thousands):
| Years Ended December 31, | Nine Months Ended September 30, |
|||||||||
| 2006 | 2007 | 2008 | 2008 | 2009 | ||||||
| (Unaudited) | ||||||||||
| Redeemable convertible preferred stock |
25,745 | 32,330 | 32,330 | 32,330 | 37,624 | |||||
| Common stock subject to repurchase |
| 58 | 25 | 30 | 10 | |||||
| Options to purchase common stock |
4,188 | 9,032 | 9,672 | 10,373 | 10,963 | |||||
| Warrants to purchase redeemable convertible preferred stock |
752 | 433 | 433 | 433 | 433 | |||||
| Warrants to purchase common stock |
55 | 59 | 59 | 59 | 59 | |||||
| Total |
30,740 | 41,912 | 42,519 | 43,225 | 49,089 | |||||
Recent Accounting Pronouncements
In June 2009, the Financial Accounting Standards Board (FASB) issued Statement of Financial Accounting Standard (SFAS) No. 168, The FASB Accounting Standards Codification and the Hierarchy of Generally Accepted Accounting Principles A Replacement of FASB Statement No. 162 (SFAS 168). SFAS 168, which is incorporated in ASC Topic 105, Generally Accepted Accounting Principles, identifies the ASC as the authoritative source of generally accepted accounting principles in the United States. Rules and interpretive releases of the SEC under federal securities laws are also sources of authoritative GAAP for SEC registrants. We adopted the provisions of the authoritative accounting guidance for the interim reporting period ended September 30, 2009 and included references to the ASC within our consolidated financial statements. The adoption had no impact on our consolidated results of operations or financial position.
In September 2006, the FASB issued SFAS No. 157, Fair Value Measurements (SFAS 157), which is incorporated in ASC Topic 820, Fair Value Measurements and Disclosures. SFAS 157 defines fair value, establishes a framework for measuring fair value and requires additional disclosures about fair value measurements. In February 2008, the FASB issued FASB Staff Position (FSP) FAS 157-1, Application of FASB Statement No. 157 to FASB Statement No. 13 and Other Pronouncements that Address Fair Value Measurements for Purpose of Lease Classification or Measurement under Statement 13, which is incorporated in ASC Topic 820, which amends SFAS 157 to exclude accounting pronouncements that address fair value measurements for purposes of lease classification or measurement under SFAS No. 13, Accounting for Leases. In February 2008, the FASB also issued FSP SFAS No. 157-2, Effective Date of FASB Statement No. 157, which is incorporated in ASC Topic 820, which delays the effective date of SFAS 157 until the first quarter of 2009 for all non-financial assets and non-financial liabilities, except for items that are recognized or disclosed at fair value in the consolidated financial statements on a recurring basis (at least annually). SFAS 157 does not require any new fair value measurements but rather eliminates inconsistencies in guidance found in various prior accounting pronouncements. In April 2009, the FASB further issued FSP SFAS No. 157-4, Determining Fair Value When the Volume and Level of Activity for the Asset or Liability Have Significantly Decreased and Identifying Transactions That Are Not Orderly (FSP SFAS 157-4), which is incorporated in ASC Topic 820. FSP SFAS 157-4 is effective for interim
F-19
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
and annual periods ending after June 15, 2009, with early adoption permitted. We adopted SFAS 157 and such adoption did not have a significant impact on our consolidated financial statements.
In December 2007, the FASB ratified EITF Issue No. 07-1, Accounting for Collaborative Agreements (EITF 07-1), which defines collaborative agreements as contractual arrangements that involve a joint operating activity. EITF 07-1, which is incorporated in ASC Topic 808, Collaborative Agreements, states that these arrangements involve two or more parties who are both active participants in the activity and that are exposed to significant risks and rewards dependent on the commercial success of the activity. EITF 07-1 provides that a company should report the effects of adoption as a change in accounting principle through retrospective application to all periods. Furthermore, it requires the parties to determine who the principal party of the arrangement is, and therefore which party must report the revenues and expenses under the collaboration, as well as specific additional disclosures in the parties financial statements. EITF 07-1 is effective for periods beginning after December 15, 2008. We adopted EITF 07-1 on January 1, 2009. The adoption did not have a significant effect on our consolidated results of operations or financial position.
In May 2009, the FASB issued SFAS No. 165, Subsequent Events (SFAS 165), which is incorporated in ASC Topic 855, Subsequent Events. The standard establishes general standards of accounting for and disclosure of events that occur after the balance sheet date but before financial statements are issued or are available to be issued. Although there is new terminology, the standard is based on the same principles as those that are currently exist in the auditing standards. The standard, which includes a new required disclosure of the date through which an entity has evaluated subsequent events, is effective for interim or annual periods ending after June 15, 2009. We adopted the provisions of this authoritative guidance in the nine month period ended September 30, 2009. The adoption had no impact on our consolidated results of operations or financial position.
In October 2009, the FASB issued Accounting Standards Update (ASU) 2009-13, which amends ASC Topic 605, Revenue Recognition, to require companies to allocate revenues in multiple-element arrangements based on an elements estimated selling price if vendor-specific or other third-party evidence of value is not available. ASU 2009-13 is effective beginning January 1, 2011. Earlier application is permitted. We are currently evaluating both the timing and the impact of the pending adoption of the ASU on our consolidated financial statements.
3. Collaborative Research and Development Agreements
Shell
In November 2006, we entered into a collaborative research agreement and a license agreement with Shell to develop biocatalysts and associated processes that use such biocatalysts. In November 2007, we entered into a new and expanded five-year collaborative research agreement and a license agreement with Shell. In March 2009, we entered into an amended collaborative research agreement and a license agreement with Shell to further expand the scope of the collaboration and allow for additional purchases of the Companys preferred stock by Shell. Shell has been a shareholder of the Company throughout all periods presented.
November 2006 Research Collaboration with Shell
In connection with the November 2006 research collaboration, Shell paid us a $2.8 million nonrefundable, up-front technology access fee, purchased 755,668 shares of our Series D redeemable convertible preferred stock at $3.97 per share for gross proceeds and an aggregate value of approximately $3.0 million, and agreed to pay us (1) research funding at specified rates per FTE working on the project
F-20
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
during the 12-month research term, (2) a $1.0 million milestone payment upon the delivery of a research report six months after the research commenced, and (3) royalties on future product sales, should such products using our technology be developed.
Under this agreement, we had a right of first negotiation to manufacture for Shell any biocatalysts developed under the collaborative research agreement if Shell decided to outsource the manufacture of such biocatalysts. In conjunction with the collaborative research agreement, Shell was issued a warrant to purchase $3.0 million of additional Series D redeemable convertible preferred stock at a price of $7.00 per share. The fair value of the warrant at issuance was determined to be $462,000 and was amortized against revenues over the twelve-month term of the collaborative research agreement. The fair value was measured using the probability-weighted expected return method. Shell exercised this warrant in full in November 2007 in connection with the new and expanded collaborative research and license agreement discussed below (see also Note 10).
In accordance with our revenue recognition policy, the $2.8 million up-front technology access fee, the $4.1 million of research funding fees and the $1.0 million milestone payment were recognized over the 12-month performance period. The $1.0 million milestone payment was concluded to not be at risk and therefore was determined to not be a substantive milestone.
November 2007 Research Collaboration with Shell
In November 2007, we entered into a five-year expanded collaborative research agreement and a license agreement with Shell. In connection with the new and expanded collaborative research agreements, Shell paid us a $20.0 million up-front exclusivity fee, purchased 3,584,428 shares of our Series E redeemable convertible preferred stock at $8.50 per share for gross proceeds of $30.5 million, and agreed to pay us (1) research funding at specified rates per FTE working on the project during the research term, (2) milestone payments upon the achievement of milestones, and (3) royalties on future product sales. The up-front exclusivity fee is refundable under certain conditions, such as a change in control in which we are acquired by a competitor of Shell. Refundability lapses ratably over a five-year period beginning on November 1, 2007, on a straight-line basis. The agreement also specifies certain minimum levels of FTE services that we must allocate to the collaboration efforts that increase over the term of the agreement. Shell has the right to terminate the collaborative research agreement upon nine months notice, subject to certain restrictions, at any time after November 2010. The term of the new and expanded agreement extends through November 2012. During the term of the agreement, we are required to act exclusively with Shell as it relates to the rights and research described in the arrangement and may not conduct research, or contract to conduct research, for another party in the field of use. Under this agreement, we also have a right of first negotiation but not an obligation to manufacture any biocatalysts developed under the collaborative research agreement if Shell decides to out-source the manufacture of such biocatalysts.
In March 2009, we entered into an amended collaborative research agreement and a license agreement with Shell. In connection with the amended collaborative research agreements, Shell purchased 3,529,412 shares of our Series F redeemable convertible preferred stock at $8.50 per share for gross proceeds of $30.0 million and agreed to pay us (1) additional research funding at specified rates per FTE working on the project during the research term and (2) additional milestone payments upon the achievement of milestones. After November 1, 2010, Shell has the right to reduce the number of funded FTEs, subject to certain limitations, with a required advance notice period ranging from 30 to 270 days, so the earliest an FTE reduction could take place would be December 2, 2010, and a subsequent period ranging from 90 to 360 days during which notices of further FTE reductions cannot be made by Shell. The length of these periods varies dependent on the number of funded FTEs reduced.
F-21
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
In accordance with our revenue recognition policy, the $20.0 million up-front exclusivity fee and the research funding fees to be received for FTE services are recognized in proportion to the actual research efforts incurred relative to the amount of total expected effort to be incurred by us over the five-year research period commencing November 2007. Milestones to be earned under this agreement have been determined to be at risk and substantive at the inception of the arrangement and are expected to be recognized upon achievement of the milestone and when collectability is reasonably assured. No milestone revenues have been recognized through December 31, 2008. We recorded milestone revenues of $0.5 million during the nine months ended September 30, 2009.
Under the agreements with Shell, we have the right to license technology from third parties that will assist us in meeting objectives under the collaboration. If a third-party technology is identified and mutually agreed upon by both parties, Shell is obligated to reimburse us for the licensing costs of the technology. In 2008, we mutually agreed to license two third-party technologies for which Shell would reimburse us the cost of the technologies. Payments made by us to the third-party providers were recorded as research and development expenses related to our collaborative research agreement with Shell. None of the acquired licenses are expected to be used in products that will be sold within the next year and the phase of the project has not reached technological feasibility. Shell reimbursed us for licensing costs of $0, $0, $6.1 million, $0 and $7.3 million for the years ended December 31, 2006, 2007 and 2008 and the nine months ended September 30, 2008 and 2009, respectively. We record these reimbursements against the costs incurred. As of December 31, 2008, $3.0 million of the reimbursements received from Shell were recorded in the consolidated balance sheet as advances from a related party and were paid to the third party in January 2009.
Other Collaborations
Pfizer
In July 2004, we entered into a multi-year collaborative research agreement and a license agreement with Pfizer to discover and develop biocatalysts, and associated processes that use such biocatalysts, in the manufacture of pharmaceutical products for Pfizer. Under the terms of these agreements, Pfizer provided us an up-front technology access fee of $2.0 million and agreed to provide research funding of approximately $8.6 million over a multi-year period. We were also eligible to receive milestone payments, a license fee if Pfizer exercised its option to acquire a non-exclusive worldwide license to our gene shuffling technology, and royalty payments based upon sales by Pfizer of products that are manufactured using our biocatalysts. The agreement was terminated in May 2007. During the term of the agreement, we received an aggregate of $600,000 of milestone payments in connection with the discovery and development of new biocatalysts on behalf of Pfizer.
In accordance with our revenue recognition policy, the $2.0 million up-front technology access fee and the research funding at specified rates per FTE working on the project were recognized over the research period under the agreement. In November 2006, following Pfizers six-month notice of termination in May 2007 of the research term, we changed our estimate of the research term from 48 to 34 months and recognized the remaining unamortized portion of the up-front payment over the reduced expected life of the research term. Research milestones were determined to be substantive and at risk at the inception of the arrangement and, as such, were recognized in the period when each milestone was achieved. Total collaborative research and development revenues recognized under this agreement was $3.7 million and $1.8 million in 2006 and 2007, respectively. No revenues were recorded under these agreements subsequent to 2007.
F-22
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
Concurrent with the execution of the multi-year collaborative research agreement and the license agreement, we also entered into a stock purchase agreement in which Pfizer purchased 1,514,645 shares of our Series C redeemable convertible preferred stock at $6.60 per share for gross proceeds of $10.0 million.
In September 2000, Maxygen, Inc. (Maxygen) extended a May 1998 agreement with Pfizer for the development of a biochemical manufacturing process for a specific pharmaceutical product. This agreement was assigned to us in connection with our initial capitalization in March 2002. The extended agreement entitled us to earn research and commercial milestones and a royalty based on a percentage of all manufacturing cost savings once the optimized commercial process was scaled up at Pfizer. During the years ended December 31, 2006, 2007 and 2008 and the nine months ended September 30, 2008 and 2009, we recognized royalty revenues related to commercial payments under this agreement in the amounts of $0.3 million, $0.3 million, $0.5 million, $0.4 million and $0.5 million, respectively.
Merck
In February 2007, we entered into a three year Catalyst License and Supply Agreement with Merck. Pursuant to the terms of the agreement, Merck may obtain enzymes from us and request that we screen the enzymes for activity in the manufacture of compounds of interest to Merck. We have granted Merck a license to use such enzymes. In connection with the agreement, Merck agreed to purchase enzyme supplies and optimization and screening services from us based on firm orders at agreed-upon rates. The minimum volume of purchases Merck is obligated to make is $4.5 million over the term of the agreement. Merck may continue to purchase supplies and services after the minimum purchase commitment period at the agreed-upon rates. Merck was also obligated to pay us additional fees upon achievement of specified milestones. The contractual term was defined as three years with licenses applicable in perpetuity. We recognize revenues from the agreement based on the amounts billed as we deliver enzyme supplies and provide the services, if all other revenue recognition criteria have been met. No amounts were billed for or recognized upon delivery of the license. During the years ended December 31, 2007 and 2008 and the nine months ended September 30, 2008 and 2009, we recognized product and collaborative research and development revenues under this agreement of $0.8 million, $2.2 million, $1.9 million and $1.8 million, respectively.
Manufacturing Collaboration
In October 2005, we entered into a technology transfer and supply agreement, which we refer to as the 2005 Agreement, with Arch Pharmalabs Ltd. (Arch), a company based in India engaged in the manufacturing and sale of active pharmaceutical ingredients, or APIs, and intermediates to pharmaceutical companies worldwide. In exchange for a $500,000 up-front payment, we granted to Arch a non-exclusive, royalty free license, with no right to grant sublicense rights, to certain of our patent rights and technology, to solely manufacture an intermediate called ATS-8 for us and on our behalf.
We also agreed to transfer technology that is necessary or useful for the manufacture of ATS-8. We recognized the fee upon delivery of the technology and the performance of certain other obligations. In exchange for a $1.5 million up-front payment, we agreed to purchase from Arch certain intermediate production quantities. The $1.5 million up-front payment was repayable by us to Arch if the specified purchases of production quantities were not met. Arch also agreed to purchase exclusively from us quantities of certain of our enzymes and an earlier intermediate used in the production of ATS-8, known as
F-23
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
ATS-5, sufficient to enable Arch to fulfill our orders for ATS-8. Subsequently, we have transferred our ATS-5 related technology to Arch for the sole purposes of manufacturing ATS-5 for our resale to Pfizer and others and for Archs use in the manufacture of ATS-8 manufactured for and on our behalf.
In August 2006, we broadened our relationship with Arch by entering into an enzyme and supply agreement, a supply agreement and a master services agreement, which we call the 2006 Agreements. The 2006 Agreements, among other things, provided biocatalytic supply specifications from us to Arch, intermediate supply from Arch to us, and services to be performed by Arch over the four year term of the agreements.
Due to the ongoing negotiations of our agreements with Arch in 2005 and 2006, we viewed the 2006 Agreements to be linked to the 2005 Agreement. We did not purchase the production volumes to earn the $1.5 million up-front payment under the 2005 Agreement so that payment was applied as consideration to the 2006 Agreements.
Under the 2006 Agreements, we agreed to pay Arch up to $1.5 million for certain chemical process and manufacturing method development services as Arch delivers them over the course of the master services agreement. For the years ended December 31, 2006, 2007 and 2008 and the nine months ended September 30, 2008 and 2009, we had paid Arch $0, $250,000, $500,000, $500,000 and $500,000, respectively, for their services under the 2006 Agreements. As of December 31, 2008 and September 30, 2009, we had a remaining liability of $750,000 and $250,000, respectively, due to Arch. We have recognized expense for these services of $156,000, $375,000, $375,000, $281,000 and $281,000 during the years ended December 31, 2006, 2007 and 2008 and the nine months ended September 30, 2008 and 2009, respectively, on a proportional basis based on quarterly manufacturing reports received from Arch.
The terms of the license prohibit Arch from using the licensed process or biocatalysts for any purpose other than manufacturing ATS-8 for sale to our affiliates. We sell the biocatalysts to Arch at cost, and Arch manufactures ATS-8 on our behalf. Arch sells ATS-8 to us at a formula-based price, which results in a fixed percentage profit share. We then directly market and sell ATS-8 to customers in the generic pharmaceutical industry, including Arch. Sales to Arch of ATS-8 are recognized net of the manufacturing costs charged by Arch. Total product and collaborative research and development revenues recorded from Arch was $219,000, $387,000, $442,000, $373,000 and $302,000 during the years ended December 31, 2006, 2007 and 2008 and the nine months ended September 30, 2008 and 2009, respectively.
In August 2008, we further expanded our relationship with Arch by entering into several enzyme and supply agreements, and product territory agreements (2008 Agreements). The 2008 Agreements, among other things, provided biocatalytic supply specifications from us to Arch, intermediate supply from Arch to us, and services to be performed by Arch over the term of the agreements for an expanded product portfolio.
4. Acquisition of BioCatalytics
On July 17, 2007, we acquired 100% of the outstanding stock of BioCatalytics for total consideration of $2.4 million. BioCatalytics offers a range of enzymes for chemical synthesis. It also provides synthesis services of metabolites and other compounds. We acquired BioCatalytics to expand our product offerings and customer relationships.
The BioCatalytics acquisition was accounted for as a business combination using the purchase method of accounting. Accordingly, the results of BioCatalytics are included in our consolidated financial statements from the date of acquisition.
F-24
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
The aggregate purchase price consisted of the following (in thousands):
| Cash consideration |
$ | 1,000 | |
| Fair value of common stock issued |
1,228 | ||
| Direct transaction costs |
219 | ||
| Total purchase price |
$ | 2,447 | |
The allocation of the total purchase price to the assets acquired and liabilities assumed based on their respective fair values at the acquisition date is as follows (in thousands):
| December 31, 2007 |
2008 Adjustments |
December 31, 2008 |
||||||||||
| Total current assets |
$ | 1,041 | $ | | $ | 1,041 | ||||||
| Property and equipment, net and other noncurrent assets |
601 | 728 | 1,329 | |||||||||
| Total liabilities assumed |
(1,227 | ) | (854 | ) | (2,081 | ) | ||||||
| Core technology |
440 | | 440 | |||||||||
| Customer relationships |
490 | | 490 | |||||||||
| Noncompete agreement |
90 | | 90 | |||||||||
| Goodwill |
1,012 | 126 | 1,138 | |||||||||
| Total purchase price |
$ | 2,447 | $ | | $ | 2,447 | ||||||
In the year ended December 31, 2008, we completed an analysis of the tax returns filed by BioCatalytics prior to our acquisition. The analysis revealed additional tax liabilities. These liabilities relate to income taxes and associated interest and penalties in pre-acquisition tax periods. As a result of the analysis, we recorded a tax liability of $0.9 million as well as $0.7 million in related assets that are discussed further below.
The merger agreement relating to the BioCatalytics acquisition provides that the former shareholder will reimburse us for his share of the tax liability associated with the final return. As a result, we have recorded a current tax liability and a corresponding receivable from the former shareholder in the amount of $0.4 million. The adjustment to other noncurrent assets comprises the $0.4 million receivable from the former shareholder and $0.3 million of deferred tax assets.
Customer relationships and core technology are being amortized over an expected useful life of five years. The non-compete agreement is being amortized over its expected useful life of three years.
F-25
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
5. Balance Sheets and Statements of Operations Details
Cash Equivalents and Marketable Securities
At December 31, 2007, cash equivalents and marketable securities, consisted of the following (in thousands):
| December 31, 2007 | |||||||||||||||
| Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Estimated Fair Value |
||||||||||||
| Money market funds |
$ | 52,125 | $ | | $ | | $ | 52,125 | |||||||
| Corporate debt obligations |
25,316 | 128 | (1 | ) | 25,443 | ||||||||||
| Asset-backed securities |
3,547 | 5 | | 3,552 | |||||||||||
| Total |
80,988 | 133 | (1 | ) | 81,120 | ||||||||||
| Less amounts classified as cash equivalents |
(52,125 | ) | | | (52,125 | ) | |||||||||
| Total marketable securities |
$ | 28,863 | $ | 133 | $ | (1 | ) | $ | 28,995 | ||||||
At December 31, 2008, cash equivalents and marketable securities, consisted of the following (in thousands):
| December 31, 2008 | |||||||||||||||
| Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Estimated Fair Value |
||||||||||||
| Money market funds |
$ | 15,992 | $ | | $ | | $ | 15,992 | |||||||
| Corporate debt obligations |
3,492 | 7 | | 3,499 | |||||||||||
| Government-sponsored enterprise securities |
11,723 | 6 | (1 | ) | 11,728 | ||||||||||
| Total |
31,207 | 13 | (1 | ) | 31,219 | ||||||||||
| Less amounts classified as cash equivalents |
(15,992 | ) | | | (15,992 | ) | |||||||||
| Total marketable securities |
$ | 15,215 | $ | 13 | $ | (1 | ) | $ | 15,227 | ||||||
At September 30, 2009, cash equivalents and marketable securities consisted of the following (in thousands):
| September 30, 2009 | |||||||||||||||
| Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Estimated Fair Value |
||||||||||||
| (Unaudited) | |||||||||||||||
| Money market funds |
$ | 17,769 | $ | | $ | | $ | 17,769 | |||||||
| U.S. Treasury obligations |
3,775 | 3 | | 3,778 | |||||||||||
| Corporate debt obligations |
1,749 | | | 1,749 | |||||||||||
| Government-sponsored enterprise securities |
28,768 | 24 | (1 | ) | 28,791 | ||||||||||
| Total |
52,061 | 27 | (1 | ) | 52,087 | ||||||||||
| Less amounts classified as cash equivalents |
(18,769 | ) | | | (18,769 | ) | |||||||||
| Total marketable securities |
$ | 33,292 | $ | 27 | $ | (1 | ) | $ | 33,318 | ||||||
All marketable securities held as of December 31, 2007, December 31, 2008 and September 30, 2009 had maturities of less than one year.
F-26
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
Inventories
Inventories consisted of the following (in thousands):
| December 31, | September 30, | ||||||||
| 2007 | 2008 | 2009 | |||||||
| (Unaudited) | |||||||||
| Raw materials |
$ | 372 | $ | 924 | $ | 1,310 | |||
| Work in process |
43 | 14 | 142 | ||||||
| Finished goods |
1,220 | 2,038 | 1,035 | ||||||
| Total inventories |
$ | 1,635 | $ | 2,976 | $ | 2,487 | |||
Property and Equipment
Property and equipment consisted of the following (in thousands):
| December 31, | ||||||||
| 2007 | 2008 | |||||||
| Laboratory equipment |
$ | 11,875 | $ | 17,558 | ||||
| Leaseholds improvements |
6,295 | 7,375 | ||||||
| Computer equipment and software |
1,105 | 1,466 | ||||||
| Office equipment and furniture |
429 | 708 | ||||||
| Construction in progress |
502 | 1,605 | ||||||
| 20,206 | 28,712 | |||||||
| Less: accumulated depreciation and amortization |
(9,107 | ) | (12,706 | ) | ||||
| Property and equipment |
$ | 11,099 | $ | 16,006 | ||||
Included in property and equipment, net is $124,000 and $75,000 of equipment under capital lease arrangements at December 31, 2007 and December 31, 2008, respectively. Included in accumulated depreciation and amortization is $121,000 and $248,000 of accumulated amortization related to equipment under capital leases at December 31, 2007 and December 31, 2008, respectively.
Intangible Assets
At December 31, 2007 and 2008, intangible assets consisted of the following (in thousands):
| December 31, 2007 | December 31, 2008 | |||||||||||||||||||
| Gross Carrying Amount |
Accumulated Amortization |
Net Carrying Value |
Gross Carrying Amount |
Accumulated Amortization |
Net Carrying Value |
|||||||||||||||
| Customer relationships |
$ | 2,850 | $ | (1,323 | ) | $ | 1,527 | $ | 2,850 | $ | (1,921 | ) | $ | 929 | ||||||
| Developed and core technology |
1,430 | (441 | ) | 989 | 1,430 | (670 | ) | 760 | ||||||||||||
| Tradename |
90 | (64 | ) | 26 | 90 | (86 | ) | 4 | ||||||||||||
| Noncompete agreements |
90 | (14 | ) | 76 | 90 | (44 | ) | 46 | ||||||||||||
| Foreign exchange adjustments |
72 | 93 | 165 | 5 | 49 | 54 | ||||||||||||||
| $ | 4,532 | $ | (1,749 | ) | $ | 2,783 | $ | 4,465 | $ | (2,672 | ) | $ | 1,793 | |||||||
F-27
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
The estimated amortization expense through the year ending December 31, 2012 is as follows at December 31, 2008 (in thousands):
| Year ending December 31: |
Cost of Product Revenues |
Selling, General and Administrative |
Total | ||||||
| 2009 |
$ | 229 | $ | 685 | $ | 914 | |||
| 2010 |
229 | 197 | 426 | ||||||
| 2011 |
229 | 98 | 327 | ||||||
| 2012 |
71 | 55 | 126 | ||||||
| $ | 758 | $ | 1,035 | $ | 1,793 | ||||
Goodwill
The changes in the carrying value of goodwill are as follows (in thousands):
| Years Ended December 31, |
||||||||
| 2007 | 2008 | |||||||
| Balance at beginning of year |
$ | 1,926 | $ | 3,099 | ||||
| Additions due to BioCatalytics acquisition |
1,012 | 126 | ||||||
| Adjustments to tax valuation allowances established in purchase accounting |
(51 | ) | | |||||
| Foreign exchange adjustments |
212 | (88 | ) | |||||
| Balance at end of year |
$ | 3,099 | $ | 3,137 | ||||
Interest Expense and Other, Net
Interest expense and other, net consisted of the following (in thousands):
| Years Ended December 31, | Nine Months Ended September 30, |
||||||||||||||||
| 2006 | 2007 | 2008 | 2008 | 2009 | |||||||||||||
| (Unaudited) | |||||||||||||||||
| Interest expense |
$ | 485 | $ | 829 | $ | 2,021 | $ | 1,603 | $ | 1,078 | |||||||
| Foreign exchange losses |
46 | 173 | 415 | 206 | 169 | ||||||||||||
| Remeasurement of redeemable convertible preferred stock warrant liabilities |
156 | 1,328 | (103 | ) | 567 | 349 | |||||||||||
| Other |
37 | 203 | 32 | 141 | (66 | ) | |||||||||||
| Interest expense and other, net |
$ | 724 | $ | 2,533 | $ | 2,365 | $ | 2,517 | $ | 1,530 | |||||||
6. Fair Value
Assets and liabilities recorded at fair value in the consolidated financial statements are categorized based upon the level of judgment associated with the inputs used to measure their fair value. Hierarchical levels which are directly related to the amount of subjectivity associated with the inputs to the valuation of these assets or liabilities are as follows:
Level 1 Inputs that are unadjusted, quoted prices in active markets for identical assets or liabilities at the measurement date.
F-28
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
Level 2 Inputs (other than quoted prices included in Level 1) that are either directly or indirectly observable for the asset or liability through correlation with market data at the measurement date and for the duration of the instruments anticipated life.
Level 3 Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities and which reflect managements best estimate of what market participants would use in pricing the asset or liability at the measurement date. Consideration is given to the risk inherent in the valuation technique and the risk inherent in the inputs to the model.
The following table presents our financial instruments that were measured at fair value on a recurring basis at December 31, 2008 by level within the fair value hierarchy (in thousands):
| December 31, 2008 | ||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||
| Financial Assets |
||||||||||||
| Money market funds |
$ | 15,992 | $ | | $ | | $ | 15,992 | ||||
| Corporate debt obligations |
| 3,499 | | 3,499 | ||||||||
| Government-sponsored enterprise securities |
| 11,728 | | 11,728 | ||||||||
| Total |
$ | 15,992 | $ | 15,227 | $ | | $ | 31,219 | ||||
| Financial Liability |
||||||||||||
| Redeemable convertible preferred stock warrant liability |
$ | | $ | | $ | 1,382 | $ | 1,382 | ||||
The change in the value of the warrant liability is summarized below (in thousands):
| Fair value at December 31, 2007 |
$ | 1,485 | ||
| Change in fair value recorded in interest expense and other, net |
(103 | ) | ||
| Fair value at December 31, 2008 |
$ | 1,382 | ||
The following table presents our financial instruments that were measured at fair value on a recurring basis at September 30, 2009 by level within the fair value hierarchy (in thousands):
| September 30, 2009 | ||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||
| (unaudited) | ||||||||||||
| Financial Assets |
||||||||||||
| Money market funds |
$ | 17,769 | $ | | $ | | $ | 17,769 | ||||
| U.S. Treasury obligations |
| 3,778 | | 3,778 | ||||||||
| Corporate debt obligations |
| 1,749 | | 1,749 | ||||||||
| Government-sponsored enterprise securities |
| 28,791 | | 28,791 | ||||||||
| Total |
$ | 17,769 | $ | 34,318 | $ | | $ | 52,087 | ||||
| Financial Liability |
||||||||||||
| Redeemable convertible preferred stock warrant liability |
$ | | $ | | $ | 1,731 | $ | 1,731 | ||||
F-29
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
The change in the value of the warrant liability is summarized below (in thousands):
| Fair value at December 31, 2008 |
$ | 1,382 | |
| Change in fair value recorded in interest expense and other, net (unaudited) |
349 | ||
| Fair value at September 30, 2009 (unaudited) |
$ | 1,731 | |
The valuation of the redeemable convertible preferred stock warrant liability is discussed in Note 10.
7. Related Party Transactions with Maxygen
Maxygen founded the Company in 2002 and remains one of the stockholders of the Company. During the years ended December 31, 2006, 2007 and 2008, and nine months ended September 30, 2009, Maxygen provided to the Company certain legal and administrative services, with total fees paid to Maxygen of $1.4 million, $652,000, $268,000, $231,000 and $70,000, respectively. At December 31, 2007, December 31, 2008 and September 30, 2009, we owed Maxygen $116,000, $26,000 and $9,000, respectively, in connection with such services.
In August 2006, Maxygen purchased 254,838 shares of Series D redeemable convertible preferred stock for $3.97 per share. Other investors not affiliated with Maxygen also purchased shares of Series D redeemable convertible preferred stock at the same price and on the same date as Maxygen. Maxygen has not subsequently purchased or been granted any additional shares.
In August 2006, we entered into an amendment to the license agreement with Maxygen. Under the amendment, we are required to pay Maxygen a fee based on a percentage of all consideration we receive from third parties related to the use of certain intellectual property owned or controlled by Maxygen in the specified field of biofuels. We also pay Maxygen a percentage of proceeds from equity financing received from such third parties. We expense all payments owed to Maxygen as they become due as collaborative research and development expenses, which we report as research and development expenses in our consolidated statements of operations. We are also obligated to reimburse up to 20% of the costs incurred by Maxygen related to the prosecution and maintenance of the patents licensed from Maxygen relating to our core technology. Further, in the event that any subsidiary or affiliate of ours develops and/or sells any energy applications using the Maxygen technology, we are obligated to transfer to Maxygen a percentage of the value of the subsidiary or affiliate that is attributable to the Maxygen technology and give Maxygen an option to acquire a percentage of the other consideration that we invest in such affiliate or subsidiary.
Currently, we pay Maxygen a fee based on our collaborative research and development agreements with Shell (see Note 3). We expensed $556,000, $7.8 million, $988,000, $579,000 and $4.2 million during the years ended December 31, 2006, 2007 and 2008 and the nine months ended September 30, 2008 and 2009, respectively. Amounts payable to Maxygen were $7.6 million, $409,000 and $314,000 at December 31, 2007, December 31, 2008 and September 30, 2009, respectively.
F-30
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
8. Financing Obligations
Financing obligations, net of debt discounts and issuance costs, consisted of the following (in thousands):
| December 31, | September 30, | |||||||||||
| 2007 | 2008 | 2009 | ||||||||||
| (unaudited) | ||||||||||||
| General Electric Capital Corporation and Oxford Finance Corporation (2007 agreement) |
$ | 14,501 | $ | 11,895 | $ | 8,460 | ||||||
| Oxford Finance Corporation (2005 agreement) |
923 | 551 | 247 | |||||||||
| Lighthouse Capital Partners V, L.P. |
858 | 103 | | |||||||||
| A German bank |
753 | 721 | 746 | |||||||||
| Total loans payable |
17,035 | 13,270 | 9,453 | |||||||||
| Lines of credit |
217 | | | |||||||||
| Capital leases |
155 | 78 | 52 | |||||||||
| 17,407 | 13,348 | 9,505 | ||||||||||
| Less: current portion |
(4,507 | ) | (5,194 | ) | (6,093 | ) | ||||||
| Financing obligations, net of current portion |
$ | 12,900 | $ | 8,154 | $ | 3,412 | ||||||
Loans Payable
In September 2007, we entered into a loan and security agreement with General Electric Capital Corporation and Oxford Finance Corporation under which we could borrow up to $15.0 million. In connection with the execution of the loan and security agreement, we incurred costs of $269,000 and, in addition, we issued the lenders a warrant to purchase 109,091 shares of Series D redeemable convertible preferred stock with an estimated fair value of $297,000, which was recorded in the consolidated balance sheet as a debt discount that is being amortized to interest expense over the life of the loans (see Note 10). During the year ended December 31, 2007, we drew down the entire $15.0 million, net of issuance costs. The loan agreement provides for 6 monthly payments of interest only and 36 monthly installments of principal and interest, with an additional 4% payment due upon final maturity of each funding. Interest accrues at 9.4% per annum. The loan is secured by substantially all of our assets except for intellectual property, and also contains covenants that, among other things, place restrictions on our use of cash including the payment of dividends, investment in subsidiaries and the purchase of capital assets. The agreement also contains events of defaults, the occurrence of which permit the lenders to declare all amounts outstanding under the loan agreement to be immediately due and payable. In addition, the lenders have the right to declare all amounts outstanding under the loan agreement to be immediately due and payable upon the occurrence of an event which has a material adverse effect on our business, assets or operations. At December 31, 2008 and September 30, 2009, we were either in compliance with the covenants of the loan and security agreement, or obtained from the lenders a waiver of noncompliance. During the years ended December 31, 2007 and December 31, 2008, we recorded interest expense of $67,000 and $250,000, respectively, for the amortization of the debt discounts related to these loans.
In October 2005, we entered into a loan agreement with Oxford Finance Corporation to borrow up to $3.0 million to be used for equipment purchases. Borrowings under the agreement to purchase equipment are secured by the equipment financed. The ability to make new borrowings under this financing agreement expired on December 31, 2006. Each borrowing is being repaid over 48-months from the date of drawdown at a fixed interest rate ranging from 9.9% to 10.7% per annum.
In February 2004, we entered into a loan agreement with Lighthouse Capital Partners V, L.P. to borrow up to $4.8 million to be used for equipment purchases and to fund working capital requirements.
F-31
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
Borrowings under this agreement to purchase equipment are secured by the equipment financed, while borrowings to fund working capital requirements are unsecured. The ability to make new borrowings under this financing agreement expired on March 31, 2005. The borrowings are being repaid over 48-months from the date of drawdown at a fixed interest rate ranging from 9.2% to 10.9% per annum and were repaid in full by January 2009.
In August 2001, JFC entered into a loan agreement with a German bank denominated in Euros and borrowed 511,000 Euro at a fixed interest rate of 7.9% per annum. The loan requires interest only payments of 10,000 Euro ($15,000, $14,000 and $15,000 as of December 31, 2007, December 31, 2008 and September 30, 2009, respectively) per quarter until September 2011, at which time the entire principal is payable in full (see Note 17).
Bridge Financing Agreement
In May 2006, we entered into a bridge financing agreement with several of our then current investors. Under the agreement, the investors loaned us a total of $4.2 million in exchange for convertible promissory notes bearing interest at an annual rate of 8.0%. With the exception of certain assets securing various notes under our other financing agreements, the notes were secured by all of our assets including our intellectual property. Commensurate with our closing of the Series D redeemable convertible preferred stock offering in August 2006, the bridge loan principal of $4.2 million plus accrued interest of $82,000 was converted into 1,078,568 shares of Series D redeemable convertible preferred stock.
In accordance with the terms of the bridge loan financing, warrants to purchase 323,569 shares of our Series D redeemable convertible preferred stock were also issued (see Note 10). The estimated fair value of the warrants of $5,000 was recorded as a debt discount with an offsetting entry to the redeemable convertible preferred stock warrant liability. As the strike price of the warrants is equal to the liquidation preference of Series D redeemable convertible preferred stock and the warrants convert to common stock warrants upon a merger or a qualified initial public offering (see Note 11), the fair value was determined on an as-converted basis using the Black-Scholes option-pricing model. The entire amount of the debt discount was amortized to interest expense during the year ended December 31, 2006. The initial allocation of proceeds received from the bridge loan financing to the warrants resulted in an embedded beneficial conversion feature in the amount of $5,000. The beneficial conversion feature was recorded as interest expense with an offsetting entry to additional paid-in capital during the year ended December 31, 2006.
Future Payments Under Financing Obligations
Future payments due for all financing obligations are as follows as of December 31, 2008 (in thousands):
|
Loans Payable |
Capital Leases | Total | ||||||||
| Years ending December 31: |
||||||||||
| 2009 |
$ | 6,351 | $ | 45 | $ | 6,396 | ||||
| 2010 |
5,975 | 33 | 6,008 | |||||||
| 2011 |
3,443 | | 3,443 | |||||||
| Total payments |
$ | 15,769 | $ | 78 | 15,847 | |||||
| Less: amount representing interest |
(2,499 | ) | ||||||||
| Outstanding principal balance of financing obligations |
13,348 | |||||||||
| Less: current portion of financing obligations |
(5,194 | ) | ||||||||
| Financing obligations, net of current portion |
$ | 8,154 | ||||||||
F-32
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
Future payments due for all financing obligations are as follows as of September 30, 2009 (in thousands, unaudited):
|
Loans Payable |
Capital Leases |
Total | ||||||||
| Three months ending December 31, 2009 |
$ | 2,299 | $ | 52 | $ | 2,351 | ||||
| Years ending December 31: |
||||||||||
| 2010 |
5,920 | | 5,920 | |||||||
| 2011 |
2,680 | | 2,680 | |||||||
| Total payments |
$ | 10,899 | $ | 52 | 10,951 | |||||
| Less: amount representing interest |
(1,446 | ) | ||||||||
| Outstanding principal balance of financing obligations |
9,505 | |||||||||
| Less: current portion of financing obligations |
(6,093 | ) | ||||||||
| Long-term portion of financing obligations |
$ | 3,412 | ||||||||
9. Commitments and Contingencies
Operating Leases
In October 2003, we entered into an operating lease agreement with a third party landlord for our facilities in Redwood City, California. The rent payments commenced in February 2004, with scheduled rent increases through the lease expiration in January 2011. During 2007 and 2008, we leased additional facilities from the same landlord adjacent to our current headquarters. The new leases expire in April 2012 and March 2013. We have an option to renew each of the three leases for a five year period. Rent expense is recognized on a straight-line basis over the term of the lease. In accordance with the terms of the lease agreement, we exercised our right to deliver a letter of credit in the amount of $450,000 in lieu of a security deposit. Provided that we have not been in default of the lease, the amount of the letter of credit will be reduced to $225,000 in February 2010. This letter of credit, which is recorded as restricted cash on the consolidated balance sheets, will be required until the termination of the lease.
Landlord allowances for leasehold improvements were $149,000, $518,000 and $54,000 for the years ended December 31, 2007, December 31, 2008 and the nine months ended September 30, 2009, respectively. We recorded these amounts as lease incentive obligations that are being amortized as a reduction of rent expense on a straight-line basis over the term of the operating lease.
We also rent facilities in Singapore, Germany and Hungary. Rent expense is being recognized on a straight-line basis over the respective terms of these leases.
We recorded a liability of $349,000 in the year ended December 31, 2007 related to asset retirement obligations from operating leases, whereby we must restore the facilities that we are renting to their original form. We are expensing the asset retirement obligation over the terms of the respective leases. We review the estimated obligation each period and we will make adjustments if our estimates change.
F-33
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
Future minimum payments under noncancellable operating leases are as follows at December 31, 2008 (in thousands):
| Lease Payments | |||
| Year ending December 31: |
|||
| 2009 |
$ | 3,058 | |
| 2010 |
2,935 | ||
| 2011 |
1,545 | ||
| 2012 |
1,213 | ||
| 2013 |
346 | ||
| $ | 9,097 | ||
Total rent expense under operating leases was $1.2 million, $2.1 million and $3.6 million, during the years ended December 31, 2006, 2007 and 2008, respectively. Deferred rent of $210,000 and $412,000 at December 31, 2007 and 2008, respectively, is included in other accrued liabilities on our consolidated balance sheets.
Litigation
We have been subject to various legal proceedings related to matters that have arisen during the ordinary course of business. Although there can be no assurance as to the ultimate disposition of these matters, we have determined, based upon the information available, that the expected outcome of these matters, individually or in the aggregate, will not have a material adverse effect on our consolidated financial position, results of operations or cash flows.
Indemnifications
We are required to recognize a liability for the fair value of any obligations we assume upon the issuance of a guarantee. Along these lines, we have certain agreements with licensors, licensees and collaborators that contain indemnification provisions. In such provisions, we typically agree to indemnify the licensor, licensee and collaborator against certain types of third party claims. The maximum amount of the indemnifications is not limited. We accrue for known indemnification issues when a loss is probable and can be reasonably estimated. There were no accruals for expenses related to indemnification issues for any periods presented.
10. Warrants
In connection with debt offerings at various times between the years ended December 31, 2004 and 2007, we issued warrants to purchase a total of 861,231 shares of our Series D redeemable convertible preferred stock and warrants to purchase a total of 58,853 shares of our common stock. The warrants are exercisable at any time during their respective terms. During the year ended December 31, 2007, a warrant to purchase 428,571 shares of Series D redeemable convertible preferred stock was exercised (see Note 3). At December 31, 2008 and September 30, 2009, the following warrants were issued and outstanding:
| Issue Date |
Class of Shares Upon Exercise |
Shares Subject to Warrants |
Exercise Price per Share |
Expiration | |||||
| February 12, 2004 |
Common | 46,176 | $ | 0.40 | February 12, 2011 | ||||
| October 25, 2005 |
Common | 9,100 | 0.70 | October 25, 2012 | |||||
| May 25, 2006 |
Series D | 323,569 | 3.97 | May 25, 2013 | |||||
| July 17, 2007 |
Common | 3,577 | 8.30 | February 9, 2016 | |||||
| September 28, 2007 |
Series D | 109,091 | 5.50 | September 28, 2017 | |||||
F-34
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
The fair value of the redeemable convertible preferred stock warrants which are recorded as liabilities in our consolidated balance sheets and are remeasured to fair value at each balance sheet date was determined using the Black-Scholes option pricing model with the following assumptions:
| December 31, | September 30, | |||||||
| 2006 | 2007 | 2008 | 2009 | |||||
| (Unaudited) | ||||||||
| Expected term in years (equals the remaining contractual term) |
1.0 - 6.4 | 5.4 - 9.8 | 4.4 - 8.7 | 3.7 - 8.0 | ||||
| Expected volatility |
48% - 49% | 44% | 57% - 65% | 72% - 75% | ||||
| Range of risk-free interest rates |
4.6% - 5.0% | 3.8% - 4.8% | 1.3% - 2.1% | 2.1% - 3.2% | ||||
| Expected dividend yield |
0.0% | 0.0% | 0.0% | 0.0% | ||||
11. Redeemable Convertible Preferred Stock
The authorized, issued and outstanding shares, aggregate liquidation preferences and carrying values of our redeemable convertible preferred stock were as follows at December 31, 2008 (in thousands):
| Number of Shares |
Aggregate Liquidation Preference |
Carrying Value |
||||||||
| Series |
Designated | Issued and Outstanding |
||||||||
| Series A |
6,000 | 6,000 | $ | 30,000 | $ | 1 | ||||
| Series B |
8,101 | 8,101 | 25,000 | 27,779 | ||||||
| Series C |
1,515 | 1,515 | 10,000 | 9,969 | ||||||
| Series D |
11,155 | 10,497 | 41,673 | 42,764 | ||||||
| Series E |
6,434 | 6,157 | 52,333 | 52,233 | ||||||
| 33,205 | 32,270 | $ | 159,006 | $ | 132,746 | |||||
The authorized, issued and outstanding shares, aggregate liquidation preferences and carrying values of our redeemable convertible preferred stock were as follows at September 30, 2009 (in thousands, unaudited):
| Number of Shares |
Aggregate Liquidation Preference |
Carrying Value |
||||||||
| Series |
Designated | Issued and Outstanding |
||||||||
| Series A |
6,000 | 6,000 | $ | 30,000 | $ | 1 | ||||
| Series B |
8,101 | 8,101 | 25,000 | 27,779 | ||||||
| Series C |
1,515 | 1,515 | 10,000 | 9,969 | ||||||
| Series D |
11,155 | 10,497 | 41,673 | 42,764 | ||||||
| Series E |
6,434 | 6,157 | 52,333 | 52,233 | ||||||
| Series F |
6,000 | 5,294 | 45,000 | 45,000 | ||||||
| 39,205 | 37,564 | $ | 204,006 | $ | 177,746 | |||||
We recorded the redeemable convertible preferred stock at fair value on the dates of issuance, net of issuance costs. We classify the redeemable convertible preferred stock outside of stockholders deficit because the shares contain redemption features that are not solely within our control. For the years ended December 31, 2007 and 2008 and the nine months ended September 30, 2009, we elected not to adjust the carrying values of the redeemable convertible preferred stock to the deemed redemption values of such shares since it is uncertain as to whether or when a liquidation event could occur. Subsequent adjustments to increase the carrying values to the ultimate redemption values will be made only when it becomes probable that such a liquidation event will occur.
F-35
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
The significant rights, privileges and preferences of our redeemable convertible preferred stock are as follows:
Voting Rights The holders of Series A through F redeemable convertible preferred stock are all entitled to one vote for each share of common stock into which such share may be converted, and the vote of the holders of a majority of our Series B, C, D, E and F redeemable convertible preferred stock (voting together as a single class and on an as-if-converted basis) is required to effect certain corporate actions. In addition, the vote of the holders of a majority of our Series D redeemable convertible preferred stock is required to affect (i) any winding up or liquidation of our Singapore subsidiary, (ii) a significant reduction in the number of employees at our Singapore subsidiary or (iii) a significant reduction in the overall technological capacity of our Singapore subsidiarys operations.
Dividends The holders of the redeemable convertible preferred stock are entitled, when, as, and if declared by the board of directors, to non-cumulative dividends of (i) $0.40 per share for Series A, (ii) $0.25 per share for Series B, (iii) $0.53 per share for Series C, (iv) $0.32 per share for Series D, (v) $0.68 per share for Series E and (vi) $0.68 per share for Series F. The Series B, C, D, E and F redeemable convertible preferred stock dividends are to be paid in advance of any distributions to the holders of Series A convertible preferred stock and common stock. The Series A convertible preferred stock dividends are to be paid in advance of any distributions to the holders of common stock. Once the redeemable convertible preferred stockholders have received their dividend preference, and in the event dividends are paid on any share of common stock, the holders of all series of redeemable convertible preferred stock are entitled to additional dividends equal to those paid or set aside to the common stockholders determined on an as-if-converted basis. No dividends have been declared or paid as of December 31, 2007, December 31, 2008 and September 30, 2009.
Liquidation In the event of any voluntary or involuntary liquidation, dissolution or winding up of our company, all of our assets available for distribution among the holders of redeemable convertible preferred stock are required to be distributed in the following order: (i) each holder of Series D, E and F redeemable convertible preferred stock is entitled to receive a liquidation preference of $3.97, $8.50 and $8.50 per share, respectively, together with any declared but unpaid dividends, before any payments can be made to holders of Series A, B and C redeemable convertible preferred stock, (ii) each holder of Series B and C redeemable convertible preferred stock is entitled to receive a liquidation preference of $3.09 and $6.60 per share, respectively, together with any declared but unpaid dividends, before any payments can be made to holders of Series A convertible preferred stock, and (iii) each holder of Series A convertible preferred stock is entitled to receive a liquidation preference of $5.00 per share, together with any declared but unpaid dividends. After payment of these preferential amounts, the remaining assets are required to be distributed ratably to holders of common stock. In the event that the assets available for distribution are insufficient to make the full per share distributions, all such assets are required to be distributed among the holders of the respective series in proportion to the full preference to which such holders would otherwise be entitled. Any of the following shall be deemed a liquidation, dissolution or winding up of our company: (1) a consolidation or merger of our company with or into any other corporation or other entity or person, or any other corporate reorganization, in which (x) we do not survive or (y) our stockholders immediately prior to such consolidation, merger or reorganization, own less than 50% of our voting power immediately after such consolidation, merger or reorganization; (2) any transaction or series of related transactions to which we are a party in which greater than 50% of our voting power is transferred; or (3) a sale, lease, exclusive license or other disposition of all or substantially all of our assets. As the holders of our redeemable convertible preferred stock may elect a majority of the members of our board of directors, and control the vote of our stockholders, a liquidation may not be in our control. Accordingly, all series of redeemable convertible preferred stock are classified outside of permanent equity.
F-36
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
From our inception through February 2005, Maxygen held a majority of our outstanding voting rights and, therefore, consolidated us as a subsidiary of Maxygen through that date. Based upon Maxygens control of us during this period, we recorded accretion adjustments to Maxygens Series B convertible preferred stock through the end of 2004, the last balance sheet date at which Maxygen retained such control. Accordingly, we adjusted the carrying value of redeemable convertible preferred stock to the deemed redemption amount during 2002, 2003 and 2004. During 2006 and 2007, our board of directors has not indicated that a deemed redemption or liquidation event, as described in the preceding paragraph, was being considered or was probable due to the reduction of Maxygens voting rights to less than a majority of our outstanding shares. Accordingly, during 2006, 2007 and 2008 and the nine months ended September 30, 2009, we did not adjust the carrying value of our Series A, B, C, D, E and F redeemable convertible preferred stock to the amounts we would have paid if a deemed redemption payment had become probable.
Conversion The holders of Series B through F redeemable convertible preferred stock have the right, at the option of the holder, at any time, to convert their shares into shares of common stock on a 1-for-1 basis, subject to adjustment for antidilution, stock splits, reclassifications and the like. The holders of the Series A convertible preferred stock have the right, at the option of the holder, at any time, to convert their shares into shares of common stock on a 1-for-1.01 basis, subject to adjustment for antidilution, stock splits, reclassifications and the like. Conversion of all outstanding redeemable convertible preferred stock is automatic (i) at any time upon the affirmative election of the holders of at least two-thirds of the then outstanding shares of the Series B, C, D, E and F, voting together as a single class and on an as-if-converted basis, or (ii) immediately upon the closing of a firmly underwritten public offering in which the gross cash proceeds to us before underwriting discounts, commissions and fees are equal to or exceed $50.0 million and our value immediately prior to the offering is equal to or exceeds $250.0 million.
Redemption At any time on or after December 31, 2013, the holders of at least a majority of the then-outstanding shares of Series B, D and E redeemable convertible preferred stock, voting or consenting together as a separate series, may require us to redeem each of these series of redeemable convertible preferred stock in three annual installments. The redemption price for each share will be payable in cash. Shares of Series B redeemable convertible preferred stock are to be redeemed at a sum equal to the applicable original issue price per share plus five percent (5%) of the original issue price per annum from the Series B original issue date until the Series D original issue date and eight percent (8%) of the original issue price per annum from the Series D original issue date until the applicable Series B redemption date, plus declared but unpaid dividends. Shares of Series D and E redeemable convertible preferred stock are to be redeemed at a sum equal to the applicable original issue price per share plus eight percent (8%) of the original issue price per annum from the original issue date until the applicable redemption date, plus declared but unpaid dividends.
12. Stockholders Deficit
In 2002, we adopted the 2002 Stock Option Plan (the Plan), under which our board of directors may issue incentive stock options, nonstatutory stock options (options that do not qualify as incentive stock options) and restricted stock to our employees, officers, directors or consultants. As of December 31, 2008 and September 30, 2009, we have reserved 15,757,642 shares of common stock for issuance under the Plan. Options granted under the Plan expire no later than 10 years from the date of grant. For incentive stock options and nonstatutory stock options, the option price shall be at least 100% and 85%, respectively, of the fair value of the common stock on the date of grant, as determined by the Board of Directors. If, at the time of a grant, the optionee directly or by attribution owns stock possessing more than 10% of the total
F-37
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
combined voting power of all of our outstanding capital stock, the exercise price for these options must be at least 110% of the fair value of the underlying common stock. Options typically vest over a four-year period at a rate of no less than 25% per year but may be granted with different vesting terms.
In the year ended December 31, 2007, our board of directors amended the Plan to allow for the early exercise of options prior to vesting. During the year ended December 31, 2007, we issued an aggregate of 130,000 unvested shares of common stock with an average exercise price of $0.81 pursuant to the early exercise of stock options. Prior to the year ended December 31, 2007, we had not issued any shares of common stock pursuant to the early exercise of stock options. The amounts received in exchange for these shares have been recorded as a liability in the accompanying consolidated balance sheet and are reclassified into equity as the shares vest. These amounts were insignificant in all periods presented.
During the nine months ended September 30, 2009, in connection with a termination of an executive officer, the Company extended the exercise period for his stock option awards to three years following the termination date, resulting in incremental stock compensation expense of $190,000. The Company also paid this officer cash severance benefits of $160,000.
The Company may also from time to time grant stock options outside the Plan. These grants and the options outstanding outside the Plan were insignificant in all periods presented.
A summary of stock option activity is as follows:
| Options Outstanding | |||||||||
|
Shares Available for Grant |
Number of Options |
Weighted-Average Exercise Price per Share |
|||||||
| December 31, 2007 |
2,446,191 | 9,032,074 | $ | 1.94 | |||||
| Authorized |
3,317,812 | | | ||||||
| Grants |
(2,105,562 | ) | 2,105,562 | 7.16 | |||||
| Exercises |
| (518,636 | ) | 0.73 | |||||
| Cancelled |
946,943 | (946,943 | ) | 4.04 | |||||
| December 31, 2008 |
4,605,384 | 9,672,057 | 2.94 | ||||||
| Authorized (unaudited) |
| | | ||||||
| Grants (unaudited) |
(2,059,495 | ) | 2,059,495 | 4.96 | |||||
| Exercises (unaudited) |
| (70,127 | ) | 1.03 | |||||
| Cancelled (unaudited) |
680,759 | (698,571 | ) | 4.25 | |||||
| September 30, 2009 (unaudited) |
3,226,648 | 10,962,854 | $ | 3.25 | |||||
The following table summarizes information about stock options outstanding and exercisable at December 31, 2008:
| Options Outstanding | Options Exercisable | |||||||||||
| Exercise Prices |
Number of Options |
Weighted-Average Remaining Contractual Term (Years) |
Weighted-Average Exercise Price per Share |
Number of Options |
Weighted-Average Exercise Price per Share |
|||||||
| $0.40-0.70 |
3,238,658 | 5.7 | $ | 0.55 | 2,937,239 | $ | 0.53 | |||||
| $1.63-1.63 |
2,559,055 | 8.0 | 1.63 | 1,295,096 | 1.63 | |||||||
| $4.47-5.79 |
2,074,482 | 8.6 | 4.58 | 704,341 | 4.59 | |||||||
| $7.00-7.90 |
1,799,862 | 9.0 | 7.20 | 189,062 | 7.01 | |||||||
| Total |
9,672,057 | 7.5 | 2.94 | 5,125,738 | 1.61 | |||||||
F-38
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
The following table summarizes information about stock options as of December 31, 2008 that are vested and are expected to vest:
| Number of Options Outstanding |
Weighted-Average Exercise Price per Share |
Weighted-Average Remaining Contractual Term (Years) |
Aggregate Intrinsic Value (In Thousands) |
|||||||
| Vested |
4,967,090 | $ | 1.47 | 6.4 | $ | 19,721 | ||||
| Expected to vest |
4,215,643 | 4.49 | 8.7 | 6,446 | ||||||
| Total vested and expected to vest |
9,182,733 | $ | 2.85 | 7.5 | $ | 26,167 | ||||
The following table summarizes information about stock options as of September 30, 2009 (unaudited) that are vested and are expected to vest:
| Number of Options Outstanding |
Weighted-Average Exercise Price per Share |
Weighted-Average Remaining Contractual Term (Years) |
Aggregate Intrinsic Value (In Thousands) |
|||||||
| Vested |
6,275,502 | $ | 2.09 | 5.9 | $ | 25,593 | ||||
| Expected to vest |
4,075,343 | 4.79 | 8.8 | 6,224 | ||||||
| Total vested and expected to vest |
10,350,845 | $ | 3.15 | 7.0 | $ | 31,817 | ||||
The weighted-average grant date fair value of options granted during the years ended December 31, 2006, 2007 and 2008 and the nine months ended September 30, 2009 was $0.78, $1.44, $3.66 and $3.28, respectively.
At December 31, 2008, exercisable options had a weighted average exercise price of $1.61 per share and an intrinsic value of $19.8 million. At September 30, 2009, exercisable options had a weighted average exercise price of $2.20 per share and an intrinsic value of $25.8 million. The aggregate intrinsic value of exercised stock options was $50,000, $869,000, $374,000 and $287,000 during the years ended December 31, 2006, 2007 and 2008 and the nine months ended September 30, 2009, respectively. The intrinsic value of stock options outstanding, exercised, exercisable and expected-to-vest is calculated based on the difference between the exercise price and the fair value of our common stock.
Stock-based compensation costs capitalized during the years ended December 31, 2006, 2007 and 2008 and the nine months ended September 30, 2008 and 2009 were insignificant. There were no stock-based compensation tax benefits during the years ended December 31, 2006, 2007 and 2008 or the nine months ended September 30, 2008 and 2009.
At December 31, 2008 and September 30, 2009, there was $9.3 million and $11.9 million, respectively, of unrecognized stock-based compensation cost which is expected to be recognized over an average period of 2.9 and 2.7 years, respectively.
Stock-Based Compensation Expense
We estimate the fair value of stock-based awards granted to employees and directors using the Black-Scholes option-pricing model. The Black-Scholes option-pricing model requires the use of highly subjective and complex assumptions to determine the fair value of stock-based awards, including the expected life of the option and expected volatility of the underlying stock over the expected life of the related grants. As a private entity, company specific historical volatility data are not available. As a result, we estimate the expected volatility based on the historical volatility of a group of unrelated public
F-39
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
companies within our industry. We will continue to consistently apply this process until a sufficient amount of historical information regarding the volatility of our own share price becomes available. Due to our limited history of grant activity, the expected life of options granted to employees is calculated using the simplified method permitted by the SEC as the average of the total contractual term of the option and its vesting period. The risk-free rate assumption was based on U.S. Treasury instruments whose terms were consistent with the terms of our stock options. The expected dividend assumption was based on our history and expectation of dividend payouts.
The following assumptions were used to estimate the fair value of our employee option grants:
| Year Ended December 31, | Nine Months Ended September 30, |
|||||||
| 2006 | 2007 | 2008 | 2009 | |||||
| (Unaudited) | ||||||||
| Weighted-average expected life (years) |
6.1 | 6.0 | 6.1 | 6.1 | ||||
| Weighted-average expected volatility |
65% | 48% | 57% | 75% | ||||
| Weighted-average risk-free interest rates |
4.2% | 4.3% | 3.2% | 2.5% | ||||
| Expected dividend yield |
0.0% | 0.0% | 0.0% | 0.0% | ||||
During the years ended December 31, 2006, 2007 and 2008 and the nine months ended September 30, 2009, we also granted options to purchase 22,500, 331,000, 30,000 and 30,000 shares of common stock, respectively, to non-employees. For options granted to non-employees, the Black-Scholes option-pricing model was applied using the following assumptions during the years ended December 31, 2006, 2007 and 2008 and the nine months ended September 30, 2009:
| Year Ended December 31, | Nine Months Ended September 30, |
|||||||
| 2006 | 2007 | 2008 | 2009 | |||||
| (Unaudited) | ||||||||
| Remaining contractual option life (years) |
8 | 9 - 10 | 7 - 9 | 6 - 10 | ||||
| Volatility |
49% - 65% | 44% - 49% | 49% | 73% - 88% | ||||
| Risk-free interest rate |
4.5% - 4.7% | 3.9% - 5.0% | 1.9% - 2.1% | 2.3% - 3.6% | ||||
| Expected dividend yield |
0.0% | 0.0% | 0.0% | 0.0% | ||||
We recognized total stock-based compensation expense during the year ended December 31, 2006 of $64,000, of which $61,000 was recorded as a general and administrative expense and $3,000 was recorded as a research and development expense. We recognized stock-based compensation expense during the year ended December 31, 2007 of $1.3 million, of which $788,000 was recorded as a general and administrative expense and $468,000 was recorded as a research and development expense. For the year ended December 31, 2008, we recognized stock-based compensation expense of $3.5 million, of which $2.0 million was recorded as general and administrative expense and $1.5 million was recorded as a research and development expense. For the nine months ended September 30, 2009, we recognized stock-based compensation expense of $3.2 million, of which $1.6 million was recorded as general and administrative expense and $1.6 million was recorded as a research and development expense.
F-40
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
Shares Reserved
Common stock reserved for future issuance is as follows (in thousands):
| December 31, 2008 | September 30, 2009 | |||
| (Unaudited) | ||||
| Conversion of redeemable convertible preferred stock |
32,330 | 37,624 | ||
| Warrants to purchase redeemable convertible preferred and common stock |
492 | 492 | ||
| Stock options: |
||||
| Outstanding |
9,672 | 10,963 | ||
| Reserved for future grants |
4,605 | 3,227 | ||
| 47,099 | 52,306 | |||
13. Income Taxes
Our loss before provision (benefit) for income taxes was as follows (in thousands):
| Years Ended December 31, | ||||||||||||
| 2006 | 2007 | 2008 | ||||||||||
| United States |
$ | (18,142 | ) | $ | (35,504 | ) | $ | (42,144 | ) | |||
| Foreign |
(656 | ) | (3,881 | ) | (2,656 | ) | ||||||
| Loss before (benefit) provision for income taxes |
$ | (18,798 | ) | $ | (39,385 | ) | $ | (44,800 | ) | |||
The tax provision (benefit) for the years ended December 31, 2006, 2007 and 2008 consists primarily of foreign tax withheld at source on royalties received from overseas and other taxes attributable to foreign operations. The components of the provision (benefit) for income taxes are as follows (in thousands):
| Years Ended December 31, | ||||||||||||
| 2006 | 2007 | 2008 | ||||||||||
| Current provision (benefit): |
||||||||||||
| Federal |
$ | | $ | | $ | 88 | ||||||
| State |
3 | 4 | 6 | |||||||||
| Foreign |
208 | 287 | 384 | |||||||||
| Total current provision |
211 | 291 | 478 | |||||||||
| Deferred provision (benefit): |
||||||||||||
| Federal |
| (131 | ) | | ||||||||
| State |
| | | |||||||||
| Foreign |
(338 | ) | (568 | ) | (151 | ) | ||||||
| Total deferred (benefit) |
(338 | ) | (699 | ) | (151 | ) | ||||||
| Total provision (benefit) |
$ | (127 | ) | $ | (408 | ) | $ | 327 | ||||
F-41
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
Reconciliation of the benefits for income taxes at the statutory rate to our provision for income taxes is as follows (in thousands):
| Years Ended December 31, | ||||||||||||
| 2006 | 2007 | 2008 | ||||||||||
| Tax benefit at federal statutory rate |
$ | (6,580 | ) | $ | (13,781 | ) | $ | (15,680 | ) | |||
| State taxes |
(859 | ) | (1,827 | ) | (1,724 | ) | ||||||
| Research and development credits |
(371 | ) | (483 | ) | (427 | ) | ||||||
| Foreign operations taxes at different rates |
(43 | ) | 1,047 | 1,144 | ||||||||
| Other nondeductible items |
147 | 560 | 3,155 | |||||||||
| Change in valuation allowance |
7,579 | 14,076 | 13,859 | |||||||||
| Provision (benefit) for income taxes |
$ | (127 | ) | $ | (408 | ) | $ | 327 | ||||
Significant components of our deferred tax assets and liabilities are as follows (in thousands):
| December 31, | ||||||||
| 2007 | 2008 | |||||||
| Deferred tax assets: |
||||||||
| Federal, state and foreign net operating loss carryforwards |
$ | 24,213 | $ | 34,690 | ||||
| Federal and state credits |
1,635 | 2,314 | ||||||
| Deferred contract revenues |
8,945 | 7,408 | ||||||
| Capitalized research and development |
323 | 209 | ||||||
| Other |
2,472 | 4,498 | ||||||
| Acquired intangible assets |
| 2,065 | ||||||
| Total deferred tax assets |
37,588 | 51,184 | ||||||
| Deferred tax liabilities: |
||||||||
| Acquired intangible assets |
(913 | ) | | |||||
| Other |
| (484 | ) | |||||
| Total deferred tax liabilities |
(913 | ) | (484 | ) | ||||
| Valuation allowance |
(36,893 | ) | (50,752 | ) | ||||
| Net deferred tax liabilities |
$ | (218 | ) | $ | (52 | ) | ||
Realization of the deferred tax assets is dependent upon future taxable income, if any, the amount and timing of which are uncertain. Accordingly, the net deferred tax assets in the United States and Germany have been fully reserved by a valuation allowance. The net valuation allowance increased by $7.6 million, $14.1 million and $13.9 million during the years ended December 31, 2006, 2007 and 2008, respectively. At such time as it is determined that it is more likely than not that the deferred tax assets are realizable, the valuation allowance will be reduced.
As of December 31, 2008, we had federal net operating losses (NOLs) of $89.0 million. We also had federal research and development tax credit carryforwards of $1.4 million. The federal NOLs will expire at various dates beginning in 2022 through 2028 if not utilized and the federal research and development tax credits will expire at various dates beginning in 2022 through 2028 if not utilized.
As of December 31, 2008, we had state NOLs of $78.2 million. We also had state research and development tax credit carryforwards of $1.5 million. The state NOLs will expire at various dates beginning in 2013 through 2028 if not utilized and the state research and development tax credits will not expire.
F-42
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
As of December 31, 2008, we had foreign NOLs of $0.7 million which do not expire.
Current federal and California tax laws include substantial restrictions on the utilization of NOLs and tax credit carryforwards in the event of an ownership change of a corporation. Accordingly, our ability to utilize NOLs and tax credit carryforwards may be limited as a result of such ownership changes. Such a limitation could result in the expiration of carryforwards before they are utilized.
We have not recorded deferred income taxes applicable to undistributed earnings of a foreign subsidiary that are indefinitely reinvested in foreign operations. Undistributed earnings amounted to $1.0 million at December 31, 2008. Generally, such earnings become subject to U.S. tax upon the remittance of dividends and under certain other circumstances.
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows (in thousands):
| December 31 | |||||||
| 2007 | 2008 | ||||||
| Balance at beginning of year |
$ | 1,661 | $ | 2,798 | |||
| Additions based on tax positions related to current year |
1,137 | 1,991 | |||||
| Additions for tax positions of prior years |
| 601 | |||||
| Reductions for tax positions of prior years |
| (267 | ) | ||||
| Balance at end of year |
$ | 2,798 | $ | 5,123 | |||
We recognize interest and penalties in income tax expense. Total interest and penalties recognized in the consolidated statement of operations was $120,000 and $169,000, respectively, during the years ended December 31, 2007 and 2008. The total unrecognized tax benefits that, if recognized, would impact our effective tax rate are $1.6 million. We do not expect any unrecognized tax benefits to be recognized within the next 12 months. We are not subject to examination by U.S. federal or state tax authorities for years prior to 2002 and foreign tax authorities for years prior to the year ended December 31, 2006.
14. 401(k) Plan
In January 2005, we implemented a 401(k) Plan covering certain employees. Currently, all of our U.S. based employees over the age of 18 are eligible to participate in the 401(k) Plan. Under the 401(k) Plan, eligible employees may elect to reduce their current compensation up to a certain annual limit and contribute these amounts to the 401(k) Plan. We may make matching or other contributions to the 401(k) Plan on behalf of eligible employees. In the years ended December 31, 2006, 2007 and 2008 and the nine months ended September 30, 2009, we did not make any contributions to the 401(k) Plan on behalf of eligible employees.
15. Restructuring Charges
In 2009, the board of directors approved and committed to plans to reduce our cost structure, which included a relocation of our operation in Germany to facilities in the U.S. and in Singapore, a rationalization of the Companys product offerings and closure of the facility in Germany, and employee terminations in Germany and the U.S. We anticipate total costs of the plans to be approximately $1.4 million, including $0.5 million in inventory write downs, $0.4 million in lease termination costs, and $0.4 million in employee severance and benefits. During the nine months ended September 30, 2009, we incurred costs of $0.2 million for employees and $0.5 million related to inventory write downs, for a total of $0.8 million. These costs were included in cost of product revenue in the consolidated statements of
F-43
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
operations. As of September 30, 2009, $0.6 million related to these expenses has been paid or charged off and the remaining $0.2 million is recorded in other accrued liabilities on the consolidated balance sheet. We expect to incur substantially all of the remaining costs of $0.6 million by December 31, 2009.
In 2008, the board of directors approved and committed to plans to reduce the Companys cost structure. The restructuring plan applied to employees and facilities worldwide. The Company expensed $1.1 million for facilities, $0.6 million for employees and $0.2 million in other costs associated with the closure of the Pasadena site for a total of $2.0 million in the year ended December 31, 2008. Restructuring expense was included in general and administrative expenses in the consolidated statements of operations. As of December 31, 2008, $0.4 million has been paid and the remaining expenses are recorded on the consolidated balance sheet in other accrued liabilities for $0.8 million and in other long-term liabilities for $0.7 million. During the nine months ended September 30, 2009, $1.3 million was paid, and $0.1 million was reversed as reduction of general and administrative expense due to a change in estimated costs of restructuring. The amounts included in other accrued liabilities on the consolidated balance sheet as of September 30, 2009 under this restructuring plan were $0.1 million.
16. Segment Reporting
Operating segments are defined as components of an enterprise about which separate financial information is available that is evaluated regularly by the chief operating decision maker, or decision making group, in deciding how to allocate resources and in assessing performance. Our chief operating decision maker is our Chief Executive Officer. The Chief Executive Officer reviews financial information presented on a consolidated basis, accompanied by information about revenues by geographic region, for purposes of allocating resources and evaluating financial performance. We have one business activity and there are no segment managers who are held accountable for operations, operating results beyond revenue goals or gross margins, or plans for levels or components below the consolidated unit level. Accordingly, we have a single reporting segment.
Operations outside of the United States consist principally of research and development and sales activities. Geographic revenues are identified by the location of the customer and consist of the following (in thousands):
| Years Ended December 31, | Nine Months Ended September 30, |
||||||||||||||
| 2006 | 2007 | 2008 | 2008 | 2009 | |||||||||||
| (Unaudited) | |||||||||||||||
| Revenues |
|||||||||||||||
| Americas(1) |
$ | 7,933 | $ | 15,010 | $ | 35,166 | $ | 22,017 | $ | 48,110 | |||||
| Europe |
2,491 | 4,005 | 8,165 | 5,934 | 6,146 | ||||||||||
| Asia |
1,703 | 6,318 | 7,147 | 3,929 | 4,414 | ||||||||||
| $ | 12,127 | $ | 25,333 | $ | 50,478 | $ | 31,880 | $ | 58,670 | ||||||
| (1) | Primarily United States. |
F-44
Table of Contents
Codexis, Inc.
Notes to Consolidated Financial Statements (Continued)
Geographic presentation of identifiable long-lived assets below shows those assets that can be directly associated with a particular geographic area and consist of the following (in thousands):
| December 31, | September 30, | |||||||||||
| 2006 | 2007 | 2008 | 2009 | |||||||||
| (Unaudited) | ||||||||||||
| Long-lived assets |
||||||||||||
| Americas(1) |
$ | 6,742 | $ | 9,470 | $ | 11,270 | $ | 13,991 | ||||
| Europe |
449 | 651 | 2,437 | 3,163 | ||||||||
| Asia |
4 | 4,780 | 5,146 | 4,460 | ||||||||
| $ | 7,195 | $ | 14,901 | $ | 18,853 | $ | 21,614 | |||||
| (1) | Primarily United States. |
17. Subsequent Events (unaudited)
We have evaluated events subsequent to September 30, 2009, the balance sheet date, through December 28, 2009, which is the date our unaudited condensed consolidated financial statements were issued.
In November 2009, we issued 235,294 additional shares of Series F redeemable convertible preferred stock at a price of $8.50 per share for gross proceeds of $2.0 million.
In November 2009, in connection with the closure of our German operations, we repaid the loan of 511,000 Euros in full due to the German bank.
On December 15, 2009, we entered into an exclusive joint development agreement with CO2 Solution, Inc. (CO2 Solution), a company based in Quebec City, Canada, whose shares are publicly traded in Canada on TSX Venture Exchange. Under the agreement, we acquired 10,000,000 common shares of CO2 Solution in a private placement for an aggregate amount of Canadian $2.0 million and paid to CO2 Solution a commitment fee of Canadian $475,000. The joint development agreement extends until January 31, 2011. Under the agreement, we expect to work with CO2 Solution to enhance to effectiveness of CO2 Solutions carbon capture processes, with CO2 Solution responsible for processes testing and enzyme delivery systems, and Codexis responsible for enzyme development and manufacturing. Each party will bear the costs it incurs under the agreement.
F-45
Table of Contents

Table of Contents
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
| Item 13. | Other Expenses of Issuance and Distribution |
The following table sets forth the fees and expenses, other than underwriting discounts and commissions, payable in connection with the registration of the common stock hereunder. All amounts are estimates except the SEC registration fee, the FINRA filing fee and The Nasdaq Global Market listing fee.
| Securities and Exchange Commission registration fee |
$ | 7,130 | |
| FINRA filing fee |
10,500 | ||
| Nasdaq Global Market listing fee |
* | ||
| Blue Sky fees and expenses |
* | ||
| Printing and engraving expenses |
* | ||
| Legal fees and expenses |
* | ||
| Accounting fees and expenses |
* | ||
| Transfer Agent and Registrar fees |
* | ||
| Miscellaneous expenses |
* | ||
| Total |
* | ||
| * | To be provided by amendment. |
| Item 14. | Indemnification of Directors and Officers |
Section 145 of the Delaware General Corporation Law provides that a corporation may indemnify its directors and officers from certain expenses in connection with legal proceedings and permits a corporation to include in its charter documents, and in agreements between the corporation and its directors and officers, provisions expanding the scope of indemnification beyond that specifically provided by this section.
The Registrants amended and restated certificate of incorporation provides for the indemnification of directors to the fullest extent permissible under Delaware law.
The Registrants amended and restated bylaws provide for the indemnification of officers, directors and third parties acting on the Registrants behalf if such persons act in good faith and in a manner reasonably believed to be in and not opposed to the Registrants best interest, and, with respect to any criminal action or proceeding, such indemnified party had no reason to believe his or her conduct was unlawful.
The Registrant has entered into indemnification agreements with each of its directors, and will enter into new indemnification agreements with each of its directors and executive officers before the completion of this offering, in addition to the indemnification provisions provided for in its charter documents. The Registrant intends to enter into indemnification agreements with any new directors and executive officers in the future.
The underwriting agreement (to be filed as Exhibit 1.1 hereto) will provide for indemnification by the underwriters of the Registrant, the Registrants executive officers and directors, and indemnification of the underwriters by the Registrant for certain liabilities, including liabilities arising under the Securities Act of 1933, as amended, in connection with matters specifically provided in writing by the underwriters for inclusion in the registration statement.
The Registrant intends to purchase and maintain insurance on behalf of any person who is or was a director or officer against any loss arising from any claim asserted against him or her and incurred by him or her in that capacity, subject to certain exclusions and limits of the amount of coverage.
II-1
Table of Contents
| Item 15. | Recent Sales of Unregistered Securities |
Since January 1, 2006, the registrant has issued and sold the following unregistered securities:
| 1. | In May 2006, the Registrant issued warrants to purchase an aggregate of 323,569 shares of its Series D convertible preferred stock at an exercise price of $3.97 per share to certain bridge lenders to the Registrant. The warrants may be exercised at any time prior to their respective termination dates, which are the 7th anniversaries of their issue dates. |
| 2. | In August and October 2006, the Registrant issued and sold 10,068,402 shares of Series D convertible preferred stock to venture capital funds and other investors at a per share price of approximately $3.97, for aggregate consideration of approximately $40.0 million. Upon completion of this offering, these shares of Series D convertible preferred stock will convert into 10,068,402 shares of the Registrants common stock. |
| 3. | In November 2006, the Registrant issued a warrant to purchase an aggregate of 428,571 shares of its Series D convertible preferred stock at an exercise price of $7.00 per share to a certain strategic partner of the Registrant. In November 2007, the warrant was exercised and the Registrant issued and sold 428,571 shares of Series D convertible preferred stock to the holder for a purchase price of approximately $3.0 million. |
| 4. | In July 2007, the Registrant issued and sold 963,423 shares of common stock to the sole shareholder of BioCatalytics, Inc. as partial consideration for the Registrants acquisition of BioCatalytics, Inc. |
| 5. | In July 2007, the Registrant converted a warrant issued by a newly-acquired subsidiary to its landlord into a warrant to purchase an aggregate of 3,577 shares of its common stock at an exercise price of $8.30 per share. The warrant may be exercised at any time prior to its termination date, which is the 10th anniversary of its issue date. |
| 6. | In September 2007, the Registrant issued warrants to purchase an aggregate of up to 109,091 shares of its Series D convertible preferred stock at an exercise price of $5.50 per share to certain lenders to the Registrant. The warrants may be exercised at any time prior to their respective termination dates, which are the 10th anniversaries of their issue dates. |
| 7. | In November and December 2007, the Registrant issued and sold 6,156,775 shares of Series E convertible preferred stock to venture capital funds and other investors at a per share price of approximately $8.50, for aggregate consideration of approximately $52.0 million. Upon completion of this offering, these shares of Series E convertible preferred stock will convert into 6,156,775 shares of the Registrants common stock. |
| 8. | In September 2008, the Registrant granted a stock option to purchase 7,812 shares of the Registrants common stock to a former director of the Registrant at an exercise price of $7.19 per share. The stock option has since been cancelled. |
| 9. | In September 2008, the Registrant granted a stock option to purchase 10,000 shares of the Registrants common stock to an employee of the Registrant at an exercise price of $4.57 per share. The stock option has since been cancelled. |
| 10. | Between March and November 2009, the Registrant issued and sold 5,529,410 shares of Series F convertible preferred stock to venture capital funds and other investors at a per share price of approximately $8.50, for aggregate consideration of approximately $47 million. Upon completion of this offering, these shares of Series F convertible preferred stock will convert into 5,529,410 shares of the Registrants common stock. |
II-2
Table of Contents
| 11. | Since January 1, 2006 through September 30, 2009, the Registrant granted stock options to purchase 10,277,570 shares of the registrants common stock at exercise prices ranging from $0.70 to $7.90 per share to employees, consultants and directors of the Registrant. Since January 1, 2006 through September 30, 2009, the Registrant had issued and sold an aggregate of 1,309,568 shares of its common stock to the Registrants employees, consultants and directors at prices ranging from $0.40 to $7.00 per share pursuant to exercises of options. |
The issuance of securities described above in paragraphs (1) through (8) and (10) were exempt from registration under the Securities Act of 1933, as amended, in reliance on Section 4(2) of the Securities Act of 1933, as amended, and Regulation D promulgated thereunder, as transactions by an issuer not involving any public offering. The purchasers of the securities in these transactions represented that they were accredited investors and that they were acquiring the securities for investment only and not with a view toward the public sale or distribution thereof. Such purchasers received written disclosures that the securities had not been registered under the Securities Act of 1933, as amended, and that any resale must be made pursuant to a registration statement or an available exemption from registration. All purchasers either received adequate financial statement or non-financial statement information about the Registrant or had adequate access, through their relationship with the Registrant, to financial statement or non-financial statement information about the Registrant. The sale of these securities was made without general solicitation or advertising.
The issuance of securities described above in paragraphs (9) and (11) were exempt from registration under the Securities Act of 1933, as amended, in reliance on Rule 701, Section 4(2) and Regulation S of the Securities Act of 1933, as amended, pursuant to compensatory benefit plans or agreements approved by the Registrants board of directors.
All certificates representing the securities issued in these transactions described in this Item 15 included appropriate legends setting forth that the securities had not been offered or sold pursuant to a registration statement and describing the applicable restrictions on transfer of the securities. There were no underwriters employed in connection with any of the transactions set forth in this Item 15.
| Item 16. | Exhibits and Financial Statement Schedules |
(a) Exhibits
| Exhibit |
Description |
|
| 1.1* | Form of Underwriting Agreement. | |
| 3.1# | Seventh Amended and Restated Certificate of Incorporation of the Registrant, as currently in effect. | |
| 3.2* | Form of Amended and Restated Certificate of Incorporation of the Registrant, to be in effect upon completion of the offering. | |
| 3.3# | Amended and Restated Bylaws of the Registrant, as currently in effect. | |
| 3.4* | Form of Amended and Restated Bylaws of the Registrant, to be in effect upon completion of the offering. | |
| 4.1* | Form of the Registrants Common Stock Certificate. | |
| 4.2# | Fifth Amended and Restated Investor Rights Agreement dated March 4, 2009. | |
| 4.3# | Form of Warrant to purchase shares of Common Stock issued in connection with the Loan and Security Agreement dated as of February 12, 2004. | |
II-3
Table of Contents
| Exhibit |
Description |
|
| 4.4# | Warrant to purchase shares of Common Stock issued to Oxford Finance Corporation dated October 25, 2005. | |
| 4.5# | Form of Warrant to purchase shares of Series D preferred stock issued in connection with the Bridge Loan Agreement dated as of May 25, 2006. | |
| 4.6# | Form of Warrant to purchase shares of Series D preferred stock issued in connection with the Loan and Security Agreement dated as of September 28, 2007. | |
| 4.7# | Warrant to purchase shares of Common Stock issued to Alexandria Equities, LLC. | |
| 4.8# | Registration Rights Agreement among the Company, Jülich Fine Chemicals GmbH and the other parties named therein, dated February 11, 2005. | |
| 4.9* | Fifth Amended and Restated Voting Agreement dated March 4, 2009. | |
| 5.1* | Opinion of Latham & Watkins LLP. | |
| 10.1A# | Loan and Security Agreement by and among the Company, General Electric Capital Corporation and Oxford Finance Corporation dated as of September 28, 2007. | |
| 10.1B# | First Amendment to Loan and Security Agreement by and among the Company, General Electric Capital Corporation and Oxford Finance Corporation dated as of November 9, 2007. | |
| 10.2A# | License Agreement by and between Maxygen, Inc. and the Company effective as of March 28, 2002 (the Maxygen License). | |
| 10.2B# | Amendment No. 1 to the Maxygen License effective as of September 13, 2002. | |
| 10.2C# | Amendment No. 2 to the Maxygen License effective as of October 1, 2002. | |
| 10.2D# | Amendment No. 3 to the Maxygen License effective as of August 22, 2006. | |
| 10.2E# | Side Letter by and between the Company and Maxygen, Inc. re: the Maxygen License dated as of February 18, 2005. | |
| 10.2F# | Side Letter by and between the Company and Maxygen, Inc. re: the Maxygen License dated as of September 11, 2007. | |
| 10.2G# | Side Letter by and between the Company and Maxygen, Inc. re: the Maxygen License dated as of September 24, 2007. | |
| 10.3A# | Amended and Restated Collaborative Research Agreement by and between the Company and Equilon Enterprises LLC dba Shell Oil Products US effective as of November 1, 2006. | |
| 10.3B# | Amendment to the Amended and Restated Collaborative Research Agreement, by and between the Company and Equilon Enterprises LLC dba Shell Oil Products US effective as of March 4, 2009. | |
| 10.4A# | Amended and Restated License Agreement by and between the Company and Equilon Enterprises LLC dba Shell Oil Products US effective as of November 1, 2007. | |
| 10.4B# | Amendment to the Amended and Restated License Agreement by and between the Company and Equilon Enterprises LLC dba Shell Oil Products US effective as of March 4, 2009. | |
| 10.5* | Collaborative Research and License Agreement by and among the Company, Iogen Energy Corporation and Equilon Enterprises LLC dba Shell Oil Products US effective as of July 10, 2009. | |
II-4
Table of Contents
| Exhibit |
Description |
|
| 10.6# | License Agreement by and among the Company, Dyadic International (USA), Inc. and Dyadic International, Inc. effective as of November 14, 2008. | |
| 10.7A* | Master Services Agreement by and between the Company and Arch Pharmalabs, Ltd. effective as of August 1, 2006 (the Master Services Agreement). | |
| 10.7B* | Amendment to Master Services Agreement effective as of August 21, 2008. |
|
| 10.7C* | Product Supply and Manufacturing Agreement (Regulated Markets) by and between the Company and Arch Pharmalabs Ltd. effective as of August 21, 2008. |
|
|
10.7D* |
Product Supply and Marketing Agreement (India) by and between Codexis Laboratories India Private Limited and Arch Pharmalabs Ltd. effective as of August 21, 2008. |
|
|
10.7E* |
Enzyme License and Development Agreement by and between the Company and Arch Pharmalabs Ltd. effective as of August 21, 2008. | |
|
10.7F* |
Enzyme Supply Agreement by and between the Company and Arch Pharmalabs Ltd. effective as of August 21, 2008. |
|
| 10.8A# | Lease Agreement by and between the Company and Metropolitan Life Insurance Company dated as of February 1, 2004. | |
| 10.8B# | Amendment to Lease Agreement by and between the Company and Metropolitan Life Insurance Company dated as of June 1, 2004. | |
| 10.8C# | Amendment to Lease Agreement by and between the Company and Metropolitan Life Insurance Company dated as of March 9, 2007. | |
| 10.8D# | Amendment to Lease Agreement by and between the Company and Metropolitan Life Insurance Company dated as of March 31, 2008. | |
| 10.9# | Master Security Agreement by and between the Company and Oxford Finance Corporation effective as of October 25, 2005. | |
| 10.10# | Codexis, Inc. 2002 Stock Plan, as amended, and Form of Stock Option Agreement. | |
| 10.11* | Codexis, Inc. 2010 Equity Incentive Award Plan and Form of Stock Option Agreement. | |
| 10.12A# | Offer Letter Agreement by and between the Company and Alan Shaw dated as of July 29, 2003. | |
| 10.13A# | Offer Letter Agreement by and between the Company and Robert S. Breuil dated as of December 22, 2005. | |
| 10.13C# | Separation Agreement by and between the Company and Robert S. Breuil dated as of June 30, 2009. | |
| 10.13D | Amendment to Separation Agreement by and between the Company and Robert S. Breuil effective as of September 25, 2009. | |
| 10.14A# | Offer Letter Agreement by and between the Company and Douglas T. Sheehy dated as of February 26, 2007. | |
| 10.15# | Offer Letter Agreement by and between Company and David L. Anton dated as of February 15, 2008. | |
| 10.16# | Employment Contract by and between the Company and Peter Seufer-Wasserthal dated as of March 6, 2006. | |
II-5
Table of Contents
| Exhibit |
Description |
|
| 10.17* | Consulting Agreement by and between the Company and Alexander A. Karsner. | |
| 10.18# | Form of Indemnification Agreement between the Company and each of its directors, as currently in effect. | |
| 10.19* | Form of Indemnification Agreement between the Company and each of its directors, officers and certain employees, to be in effect before the completion of the offering. | |
| 10.20 | Offer Letter Agreement by and between the Company and Robert J. Lawson dated as of October 16, 2009. | |
| 10.21 | 2008 Executive Incentive Compensation Plan. | |
| 10.22 | 2009 Executive Incentive Compensation Plan. | |
| 10.23 | Form of Change of Control Severance Agreement between the Company and certain of its officers. | |
| 21# | List of Subsidiaries. | |
| 23.1 | Consent of independent registered public accounting firm. | |
| 23.2* | Consent of Latham & Watkins LLP (included in Exhibit 5.1). | |
| 24.1# | Power of Attorney (see page II-8 of the original filing of this Form S-1). | |
| * | To be filed by amendment. |
| | Certain portions have been omitted pursuant to a confidential treatment request. Omitted information has been filed separately with the SEC. |
| # | Previously filed. |
(b) Financial Statement Schedules
Schedules not listed above have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto.
| Item 17. | Undertakings |
Insofar as indemnification for liabilities arising under the Securities Act of 1933, as amended, may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933, as amended, and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933, as amended, and will be governed by the final adjudication of such issue.
The Registrant hereby undertakes that:
(a) The Registrant will provide to the underwriters at the closing as specified in the underwriting agreement, certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
II-6
Table of Contents
(b) For purposes of determining any liability under the Securities Act of 1933, as amended, the information omitted from a form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in the form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act of 1933, as amended, shall be deemed to be part of this registration statement as of the time it was declared effective.
(c) For the purpose of determining any liability under the Securities Act of 1933, as amended, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-7
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the Registrant has duly caused this Amendment No. 2 to the Registration Statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Redwood City, State of California, on the 1st day of February, 2010.
| CODEXIS, INC. | ||
| By: | /S/ ALAN SHAW |
|
| Alan Shaw President and Chief Executive Officer |
||
Pursuant to the requirements of the Securities Act of 1933, as amended, this Amendment No. 2 to the Registration Statement has been signed by the following persons in the capacities indicated below.
| Signature |
Title |
Date |
||
| /S/ ALAN SHAW Alan Shaw |
President and Chief Executive Officer, Director (Principal Executive Officer) |
February 1, 2010 | ||
| /S/ ROBERT J. LAWSON Robert J. Lawson |
Senior Vice President and Chief Financial Officer (Principal Financial and Accounting Officer) |
February 1, 2010 |
||
| * Thomas R. Baruch |
Chairman of the Board of Directors | February 1, 2010 |
||
| * Alexander A. Karsner |
Director |
February 1, 2010 |
||
| * Bernard J. Kelley |
Director | February 1, 2010 |
||
| * Bruce Pasternack |
Director | February 1, 2010 |
||
| * Chris Streng |
Director | February 1, 2010 |
||
| * James R. Sulat |
Director | February 1, 2010 |
||
| * Dennis P. Wolf |
Director | February 1, 2010 |
||
| * Mun Yew Wong |
Director | February 1, 2010 |
||
| *By: | /S/ ALAN SHAW | February 1 2010 | ||||
| Alan Shaw Attorney-in-fact |
||||||
II-8
Table of Contents
EXHIBIT INDEX
| Exhibit |
Description |
|
| 1.1* | Form of Underwriting Agreement. | |
| 3.1# | Seventh Amended and Restated Certificate of Incorporation of the Registrant, as currently in effect. | |
| 3.2* | Form of Amended and Restated Certificate of Incorporation of the Registrant, to be in effect upon completion of the offering. | |
| 3.3# | Amended and Restated Bylaws of the Registrant, as currently in effect. | |
| 3.4* | Form of Amended and Restated Bylaws of the Registrant, to be in effect upon completion of the offering. | |
| 4.1* | Form of the Registrants Common Stock Certificate. | |
| 4.2# | Fifth Amended and Restated Investor Rights Agreement dated March 4, 2009. | |
| 4.3# | Form of Warrant to purchase shares of Common Stock issued in connection with the Loan and Security Agreement dated as of February 12, 2004. | |
| 4.4# | Warrant to purchase shares of Common Stock issued to Oxford Finance Corporation dated October 25, 2005. | |
| 4.5# | Form of Warrant to purchase shares of Series D preferred stock issued in connection with the Bridge Loan Agreement dated as of May 25, 2006. | |
| 4.6# | Form of Warrant to purchase shares of Series D preferred stock issued in connection with the Loan and Security Agreement dated as of September 28, 2007. | |
| 4.7# | Warrant to purchase shares of Common Stock issued to Alexandria Equities, LLC. | |
| 4.8# | Registration Rights Agreement among the Company, Jülich Fine Chemicals GmbH and the other parties named therein, dated February 11, 2005. | |
| 4.9* | Fifth Amended and Restated Voting Agreement dated March 4, 2009. | |
| 5.1* | Opinion of Latham & Watkins LLP. | |
| 10.1A# | Loan and Security Agreement by and among the Company, General Electric Capital Corporation and Oxford Finance Corporation dated as of September 28, 2007. | |
| 10.1B# | First Amendment to Loan and Security Agreement by and among the Company, General Electric Capital Corporation and Oxford Finance Corporation dated as of November 9, 2007. | |
| 10.2A# | License Agreement by and between Maxygen, Inc. and the Company effective as of March 28, 2002 (the Maxygen License). | |
| 10.2B# | Amendment No. 1 to the Maxygen License effective as of September 13, 2002. | |
| 10.2C# | Amendment No. 2 to the Maxygen License effective as of October 1, 2002. | |
| 10.2D# | Amendment No. 3 to the Maxygen License effective as of August 22, 2006. | |
| 10.2E# | Side Letter by and between the Company and Maxygen, Inc. re: the Maxygen License dated as of February 18, 2005. | |
| 10.2F# | Side Letter by and between the Company and Maxygen, Inc. re: the Maxygen License dated as of September 11, 2007. | |
| 10.2G# | Side Letter by and between the Company and Maxygen, Inc. re: the Maxygen License dated as of September 24, 2007. | |
Table of Contents
| Exhibit |
Description |
|
| 10.3A# | Amended and Restated Collaborative Research Agreement by and between the Company and Equilon Enterprises LLC dba Shell Oil Products US effective as of November 1, 2006. | |
| 10.3B# | Amendment to the Amended and Restated Collaborative Research Agreement by and between the Company and Equilon Enterprises LLC dba Shell Oil Products US effective as of March 4, 2009. | |
| 10.4A# | Amended and Restated License Agreement by and between the Company and Equilon Enterprises LLC dba Shell Oil Products US effective as of November 1, 2007. | |
| 10.4B# | Amendment to the Amended and Restated License Agreement by and between the Company and Equilon Enterprises LLC dba Shell Oil Products US effective as of March 4, 2009. | |
| 10.5* | Collaborative Research and License Agreement by and among the Company, Iogen Energy Corporation and Equilon Enterprises LLC dba Shell Oil Products US effective as of July 10, 2009. | |
| 10.6# | License Agreement by and among the Company, Dyadic International (USA), Inc. and Dyadic International, Inc. effective as of November 14, 2008. | |
| 10.7A* | Master Services Agreement by and between the Company and Arch Pharmalabs, Ltd. effective as of August 1, 2006 (the Master Services Agreement). | |
| 10.7B* | Amendment to Master Services Agreement effective as of August 21, 2008. | |
| 10.7C* | Product Supply and Manufacturing Agreement (Regulated Markets) by and between the Company and Arch Pharmalabs Ltd. effective as of August 21, 2008. | |
| 10.7D* | Product Supply and Marketing Agreement (India) by and between Codexis Laboratories India Private Limited and Arch Pharmalabs Ltd. effective as of August 21, 2008. | |
| 10.7E* | Enzyme License and Development Agreement by and between the Company and Arch Pharmalabs Ltd. effective as of August 21, 2008. | |
| 10.7F* | Enzyme Supply Agreement by and between the Company and Arch Pharmalabs Ltd. effective as of August 21, 2008. | |
| 10.8A# | Lease Agreement by and between the Company and Metropolitan Life Insurance Company dated as of February 1, 2004. | |
| 10.8B# | Amendment to Lease Agreement by and between the Company and Metropolitan Life Insurance Company dated as of June 1, 2004. | |
| 10.8C# | Amendment to Lease Agreement by and between the Company and Metropolitan Life Insurance Company dated as of March 9, 2007. | |
| 10.8D# | Amendment to Lease Agreement by and between the Company and Metropolitan Life Insurance Company dated as of March 31, 2008. | |
| 10.9# | Master Security Agreement by and between the Company and Oxford Finance Corporation effective as of October 25, 2005. | |
| 10.10# | Codexis, Inc. 2002 Stock Plan, as amended, and Form of Stock Option Agreement. | |
| 10.11* | Codexis, Inc. 2010 Equity Incentive Award Plan and Form of Stock Option Agreement. | |
| 10.12A# | Offer Letter Agreement by and between the Company and Alan Shaw dated as of July 29, 2003. | |
| 10.13A# | Offer Letter Agreement by and between the Company and Robert S. Breuil dated as of December 22, 2005. | |
Table of Contents
| Exhibit |
Description |
|
| 10.13C# | Separation Agreement by and between the Company and Robert S. Breuil dated as of June 30, 2009. | |
| 10.13D | Amendment to Separation Agreement by and between the Company and Robert S. Breuil effective as of September 25, 2009. | |
| 10.14A# | Offer Letter Agreement by and between the Company and Douglas T. Sheehy dated as of February 26, 2007. | |
| 10.15# | Offer Letter Agreement by and between Company and David L. Anton dated as of February 15, 2008. | |
| 10.16# | Employment Contract by and between the Company and Peter Seufer-Wasserthal dated as of March 6, 2006. | |
| 10.17* | Consulting Agreement by and between the Company and Alexander A. Karsner. | |
| 10.18# | Form of Indemnification Agreement between the Company and each of its directors, as currently in effect. | |
| 10.19* | Form of Indemnification Agreement between the Company and each of its directors, officers and certain employees, to be in effect before the completion of the offering. | |
| 10.20 | Offer Letter Agreement by and between the Company and Robert J. Lawson dated as of October 16, 2009. | |
| 10.21 | 2008 Executive Incentive Compensation Plan. | |
| 10.22 | 2009 Executive Incentive Compensation Plan. | |
| 10.23 | Form of Change of Control Severance Agreement between the Company and certain of its officers. | |
| 21# | List of Subsidiaries | |
| 23.1 | Consent of independent registered public accounting firm. | |
| 23.2* | Consent of Latham & Watkins LLP (included in Exhibit 5.1). | |
| 24.1# | Power of Attorney (see page II-8 of the original filing of this Form S-1). | |
| * | To be filed by amendment. |
| | Certain portions have been omitted pursuant to a confidential treatment request. Omitted information has been filed separately with the SEC. |
| # | Previously filed. |